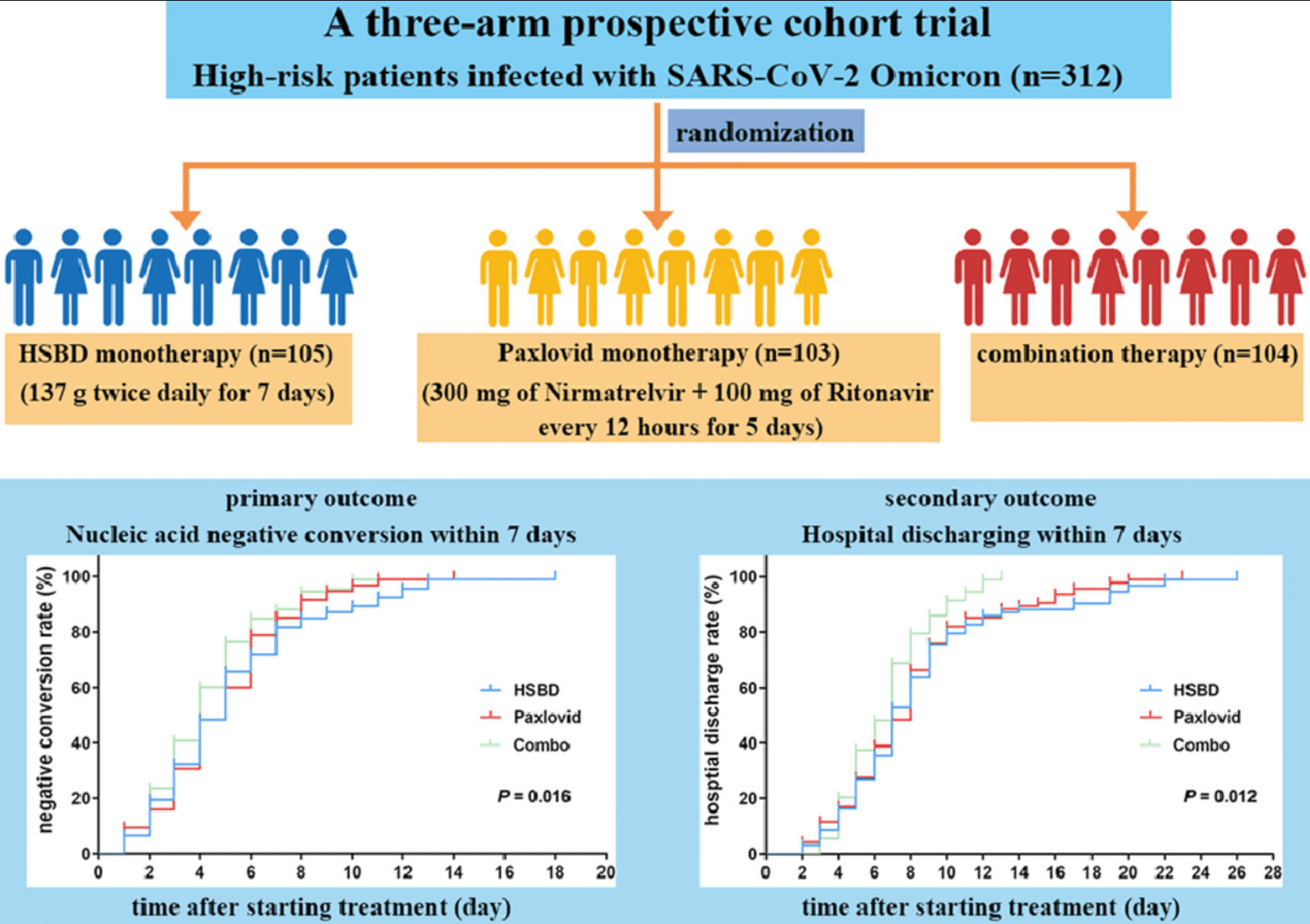
Efficacy and safety of Huashi Baidu granule plus Nirmatrelvir-Ritonavir combination therapy in patients with high-risk factors infected with Omicron (B.1.1.529): A multi-arm single-center, open-label, randomized controlled trial
et al., Phytomedicine, doi:10.1016/j.phymed.2023.155025, ChiCTR2200059390, Aug 2023
RCT 312 hospitalized COVID-19 patients in China, showing no significant difference between paxlovid and Huashi Baidu treatment. Combination therapy showed improved results to either treatment alone.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments1.
|
risk of severe case, 1.9% higher, RR 1.02, p = 1.00, treatment 3 of 103 (2.9%), control 3 of 105 (2.9%).
|
|
risk of no hospital discharge, 6.3% higher, RR 1.06, p = 0.78, treatment 49 of 103 (47.6%), control 47 of 105 (44.8%).
|
|
risk of no viral clearance, 12.0% lower, HR 0.88, p = 0.33, treatment 30 of 103 (29.1%), control 34 of 105 (32.4%), NNT 31.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Yu et al., 16 Aug 2023, Randomized Controlled Trial, China, peer-reviewed, 18 authors, study period 18 April, 2022 - 5 June, 2022, this trial compares with another treatment - results may be better when compared to placebo, trial ChiCTR2200059390.
DOI record:
{
"DOI": "10.1016/j.phymed.2023.155025",
"ISSN": [
"0944-7113"
],
"URL": "http://dx.doi.org/10.1016/j.phymed.2023.155025",
"alternative-id": [
"S0944711323003860"
],
"article-number": "155025",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Efficacy and safety of Huashi Baidu granule plus Nirmatrelvir-Ritonavir combination therapy in patients with high-risk factors infected with Omicron (B.1.1.529): A multi-arm single-center, open-label, randomized controlled trial"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Phytomedicine"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.phymed.2023.155025"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2023 Published by Elsevier GmbH."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-4100-8415",
"affiliation": [],
"authenticated-orcid": false,
"family": "Yu",
"given": "Zhuo",
"sequence": "first"
},
{
"affiliation": [],
"family": "Zheng",
"given": "Yanxi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chen",
"given": "Bowu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lv",
"given": "Jia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhu",
"given": "Xiaojun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shang",
"given": "Binyi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Xv",
"given": "Yuping",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tao",
"given": "Ru",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yang",
"given": "Yanbing",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cong",
"given": "Jun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Dan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wu",
"given": "Huan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Qv",
"given": "Wenchao",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Xiyi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Xv",
"given": "Chengbin",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9820-7111",
"affiliation": [],
"authenticated-orcid": false,
"family": "Feng",
"given": "Hai",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yuan",
"given": "Weian",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7276-9810",
"affiliation": [],
"authenticated-orcid": false,
"family": "Gao",
"given": "Yueqiu",
"sequence": "additional"
}
],
"container-title": "Phytomedicine",
"container-title-short": "Phytomedicine",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2023,
8,
16
]
],
"date-time": "2023-08-16T15:39:30Z",
"timestamp": 1692200370000
},
"deposited": {
"date-parts": [
[
2023,
8,
26
]
],
"date-time": "2023-08-26T10:21:02Z",
"timestamp": 1693045262000
},
"indexed": {
"date-parts": [
[
2023,
8,
27
]
],
"date-time": "2023-08-27T14:11:10Z",
"timestamp": 1693145470222
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
11
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
11,
1
]
],
"date-time": "2023-11-01T00:00:00Z",
"timestamp": 1698796800000
}
},
{
"URL": "https://doi.org/10.15223/policy-017",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
11,
1
]
],
"date-time": "2023-11-01T00:00:00Z",
"timestamp": 1698796800000
}
},
{
"URL": "https://doi.org/10.15223/policy-037",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
11,
1
]
],
"date-time": "2023-11-01T00:00:00Z",
"timestamp": 1698796800000
}
},
{
"URL": "https://doi.org/10.15223/policy-012",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
11,
1
]
],
"date-time": "2023-11-01T00:00:00Z",
"timestamp": 1698796800000
}
},
{
"URL": "https://doi.org/10.15223/policy-029",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
11,
1
]
],
"date-time": "2023-11-01T00:00:00Z",
"timestamp": 1698796800000
}
},
{
"URL": "https://doi.org/10.15223/policy-004",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
11,
1
]
],
"date-time": "2023-11-01T00:00:00Z",
"timestamp": 1698796800000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S0944711323003860?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S0944711323003860?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "155025",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2023,
11
]
]
},
"published-print": {
"date-parts": [
[
2023,
11
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.3389/fmed.2022.849217",
"article-title": "The biological functions and clinical significance of SARS-CoV-2 variants of corcern",
"author": "Akkiz",
"doi-asserted-by": "crossref",
"journal-title": "Front. Med.",
"key": "10.1016/j.phymed.2023.155025_bib0001",
"volume": "9",
"year": "2022"
},
{
"article-title": "Emerging variants of SARS-CoV-2 and novel therapeutics against coronavirus (COVID-19), updated 2022 May 12 ed",
"author": "Aleem",
"key": "10.1016/j.phymed.2023.155025_bib0002",
"series-title": "StatPearls",
"year": "2022"
},
{
"DOI": "10.1126/science.1085658",
"article-title": "Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs",
"author": "Anand",
"doi-asserted-by": "crossref",
"first-page": "1763",
"journal-title": "Science",
"key": "10.1016/j.phymed.2023.155025_bib0003",
"volume": "300",
"year": "2003"
},
{
"DOI": "10.21037/apm-20-1759",
"article-title": "The pharmacological mechanism of Huashi Baidu Formula for the treatment of COVID-19 by combined network pharmacology and molecular docking",
"author": "Cai",
"doi-asserted-by": "crossref",
"first-page": "3864",
"journal-title": "Ann. Palliat Med.",
"key": "10.1016/j.phymed.2023.155025_bib0004",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1136/bmj.310.6977.452",
"article-title": "The number needed to treat: a clinically useful measure of treatment effect",
"author": "Cook",
"doi-asserted-by": "crossref",
"first-page": "452",
"journal-title": "BMJ",
"key": "10.1016/j.phymed.2023.155025_bib0005",
"volume": "310",
"year": "1995"
},
{
"DOI": "10.1016/j.drup.2021.100794",
"article-title": "An update on drugs with therapeutic potential for SARS-CoV-2 (COVID-19) treatment",
"author": "Drozdzal",
"doi-asserted-by": "crossref",
"journal-title": "Drug Resist. Updat.",
"key": "10.1016/j.phymed.2023.155025_bib0006",
"volume": "59",
"year": "2021"
},
{
"DOI": "10.1136/bmj.o1037",
"article-title": "COVID-19: what is the evidence for the antiviral Paxlovid?",
"author": "Extance",
"doi-asserted-by": "crossref",
"first-page": "o1037",
"journal-title": "BMJ",
"key": "10.1016/j.phymed.2023.155025_bib0007",
"volume": "377",
"year": "2022"
},
{
"DOI": "10.1016/j.bbrc.2020.10.102",
"article-title": "Evolution patterns of SARS-CoV-2: snapshot on its genome variants",
"author": "Giovanetti",
"doi-asserted-by": "crossref",
"first-page": "88",
"journal-title": "Biochem. Biophys. Res. Commun.",
"key": "10.1016/j.phymed.2023.155025_bib0008",
"volume": "538",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in hospitalized patients with COVID-19",
"author": "Horby",
"doi-asserted-by": "crossref",
"first-page": "693",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.phymed.2023.155025_bib0009",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.17023",
"article-title": "Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis",
"author": "Sterne",
"doi-asserted-by": "crossref",
"first-page": "1330",
"journal-title": "JAMA",
"key": "10.1016/j.phymed.2023.155025_bib0010",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral Nirmatrelvir for high-risk, nonhospitalized adults with COVID-19",
"author": "Hammond",
"doi-asserted-by": "crossref",
"first-page": "1397",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.phymed.2023.155025_bib0011",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1111/febs.12936",
"article-title": "From SARS to MERS: crystallographic studies on coronaviral proteases enable antiviral drug design",
"author": "Hilgenfeld",
"doi-asserted-by": "crossref",
"first-page": "4085",
"journal-title": "FEBS J.",
"key": "10.1016/j.phymed.2023.155025_bib0012",
"volume": "281",
"year": "2014"
},
{
"DOI": "10.1001/jama.2019.3087",
"article-title": "Reporting of multi-arm parallel-group randomized trials: extension of the CONSORT 2010 statement",
"author": "Juszczak",
"doi-asserted-by": "crossref",
"first-page": "1610",
"journal-title": "JAMA",
"key": "10.1016/j.phymed.2023.155025_bib0013",
"volume": "321",
"year": "2019"
},
{
"DOI": "10.1007/s11684-020-0801-x",
"article-title": "The role played by traditional Chinese medicine in preventing and treating COVID-19 in China",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "681",
"journal-title": "Front. Med.",
"key": "10.1016/j.phymed.2023.155025_bib0014",
"volume": "14",
"year": "2020"
},
{
"DOI": "10.1016/j.phymed.2021.153671",
"article-title": "Combination of Hua Shi Bai Du granule (Q-14) and standard care in the treatment of patients with coronavirus disease 2019 (COVID-19): a single-center, open-label, randomized controlled trial",
"author": "Liu",
"doi-asserted-by": "crossref",
"journal-title": "Phytomedicine",
"key": "10.1016/j.phymed.2023.155025_bib0015",
"volume": "91",
"year": "2021"
},
{
"DOI": "10.1186/s13020-020-00375-1",
"article-title": "Reflections on treatment of COVID-19 with traditional Chinese medicine",
"author": "Luo",
"doi-asserted-by": "crossref",
"first-page": "94",
"journal-title": "Chin. Med.",
"key": "10.1016/j.phymed.2023.155025_bib0016",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.1136/bmj.n2713",
"article-title": "COVID-19: Pfizer's paxlovid is 89% effective in patients at risk of serious illness, company reports",
"author": "Mahase",
"doi-asserted-by": "crossref",
"first-page": "n2713",
"journal-title": "BMJ",
"key": "10.1016/j.phymed.2023.155025_bib0017",
"volume": "375",
"year": "2021"
},
{
"DOI": "10.1126/science.abl4784",
"article-title": "An oral SARS-CoV-2 M(pro) inhibitor clinical candidate for the treatment of COVID-19",
"author": "Owen",
"doi-asserted-by": "crossref",
"first-page": "1586",
"journal-title": "Science",
"key": "10.1016/j.phymed.2023.155025_bib0018",
"volume": "374",
"year": "2021"
},
{
"DOI": "10.1016/j.virusres.2020.198057",
"article-title": "Potential drugs for the treatment of the novel coronavirus pneumonia (COVID-19) in China",
"author": "Pan",
"doi-asserted-by": "crossref",
"journal-title": "Virus Res.",
"key": "10.1016/j.phymed.2023.155025_bib0019",
"volume": "286",
"year": "2020"
},
{
"article-title": "Traditional Chinese medicine for COVID-19 treatment",
"author": "Ren",
"journal-title": "Pharmacol. Res.",
"key": "10.1016/j.phymed.2023.155025_bib0020",
"volume": "155",
"year": "2020"
},
{
"DOI": "10.1016/j.phymed.2020.153367",
"article-title": "Efficacy and safety of Chinese herbal medicine versus Lopinavir-Ritonavir in adult patients with coronavirus disease 2019: a non-randomized controlled trial",
"author": "Shi",
"doi-asserted-by": "crossref",
"journal-title": "Phytomedicine",
"key": "10.1016/j.phymed.2023.155025_bib0021",
"volume": "81",
"year": "2021"
},
{
"DOI": "10.1101/2020.04.13.038687",
"doi-asserted-by": "crossref",
"key": "10.1016/j.phymed.2023.155025_bib0022",
"unstructured": "Su, H., Yao, S., Zhao, W., Li, M., Liu, J., Shang, W., Xie, H., Ke, C., Gao, M., Yu, K., Liu, H., Shen, J., Tang, W., Zhang, L., Zuo, J., Jiang, H., Bai, F., Wu, Y., Ye, Y., Xu, Y., 2020. Discovery of baicalin and baicalein as novel, natural product inhibitors of SARS-CoV-2 3CL protease in vitro. Biorxiv."
},
{
"DOI": "10.1080/03639045.2020.1788070",
"article-title": "Network pharmacology and molecular docking analysis on molecular targets and mechanisms of Huashi Baidu formula in the treatment of COVID-19",
"author": "Tao",
"doi-asserted-by": "crossref",
"first-page": "1345",
"journal-title": "Drug Dev. Ind. Pharm.",
"key": "10.1016/j.phymed.2023.155025_bib0023",
"volume": "46",
"year": "2020"
},
{
"DOI": "10.1016/j.antiviral.2022.105252",
"article-title": "Remdesivir, Molnupiravir and Nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern",
"author": "Vangeel",
"doi-asserted-by": "crossref",
"journal-title": "Antiviral Res.",
"key": "10.1016/j.phymed.2023.155025_bib0024",
"volume": "198",
"year": "2022"
},
{
"article-title": "Study of molecular mechanism of Huashibaidu decoction on COVID-19 based on network pharmacology and molecular docking technology",
"author": "Xie",
"first-page": "28",
"journal-title": "Pharmcol. Clin. Chin. Mater. Med.",
"key": "10.1016/j.phymed.2023.155025_bib0025",
"volume": "36",
"year": "2020"
},
{
"DOI": "10.1016/S2213-2600(20)30076-X",
"article-title": "Pathological findings of COVID-19 associated with acute respiratory distress syndrome",
"author": "Xu",
"doi-asserted-by": "crossref",
"first-page": "420",
"journal-title": "Lancet Respir. Med.",
"key": "10.1016/j.phymed.2023.155025_bib0026",
"volume": "8",
"year": "2020"
},
{
"article-title": "Material basis research of anti-COVID-19 Huashibaidu granule gormula",
"author": "Yang",
"first-page": "672",
"journal-title": "Mod. Chin. Med.",
"key": "10.1016/j.phymed.2023.155025_bib0027",
"volume": "22",
"year": "2020"
}
],
"reference-count": 27,
"references-count": 27,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S0944711323003860"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Complementary and alternative medicine",
"Drug Discovery",
"Pharmaceutical Science",
"Pharmacology",
"Molecular Medicine"
],
"subtitle": [],
"title": "Efficacy and safety of Huashi Baidu granule plus Nirmatrelvir-Ritonavir combination therapy in patients with high-risk factors infected with Omicron (B.1.1.529): A multi-arm single-center, open-label, randomized controlled trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "120"
}