A randomized clinical trial study on the efficacy and safety of adalimumab and methylprednisolone pulse therapy in the treatment of COVID-19 patients with acute respiratory distress syndrome
et al., Immunopathologia Persa, doi:10.34172/ipp.2022.30322, IRCT20200406046963N2, Dec 2021
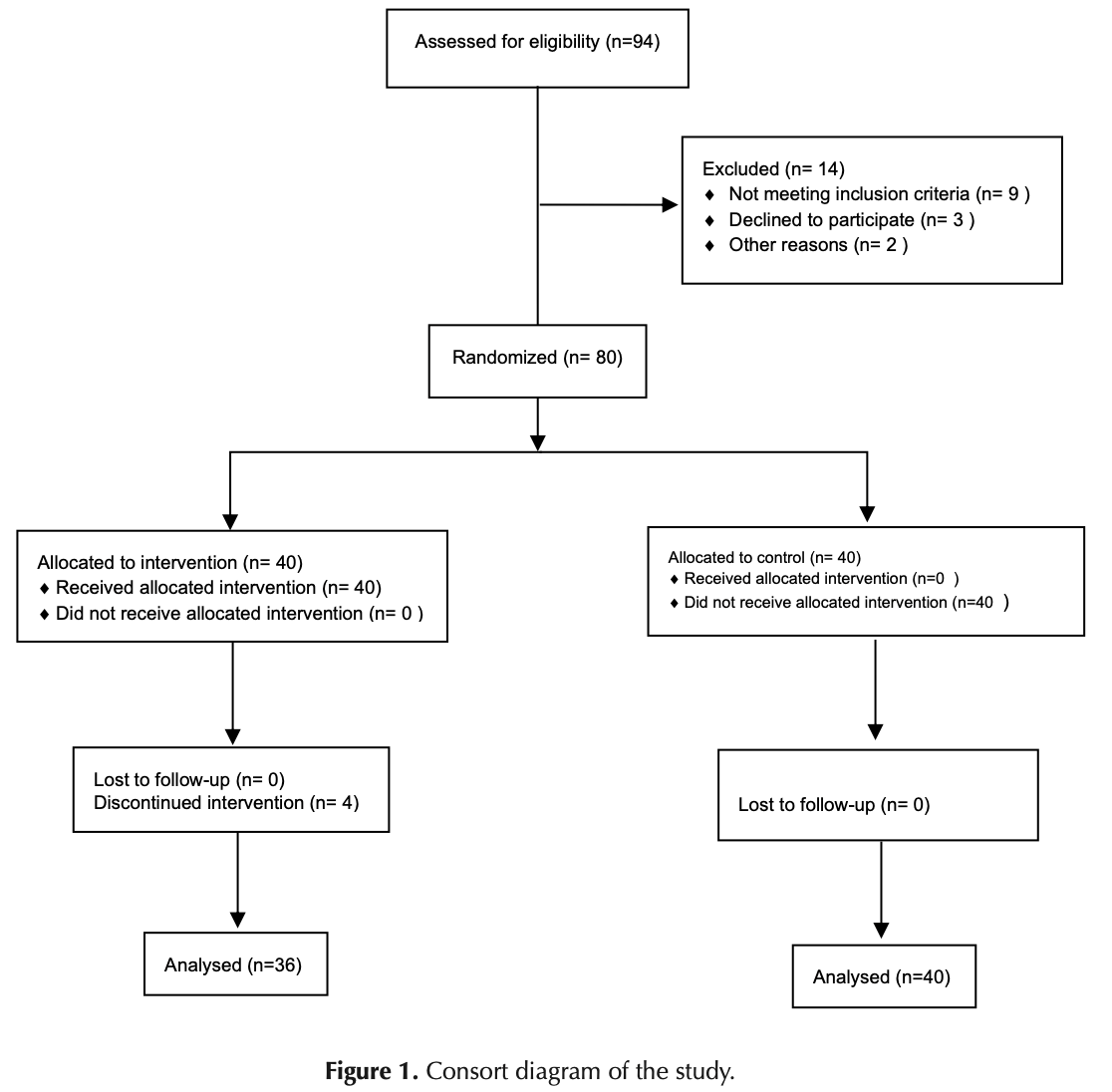
RCT 80 ICU patients with moderate to severe COVID-19 showing reduced hospital stay with adalimumab plus methylprednisolone compared to methylprednisolone alone, however authors excluded patients that died without reporting how many died. 4 patients were excluded in the treatment group, while no patients were excluded in the control group, suggesting that 2-4 treatment patients died, while no control patients died.
Yazdani et al., 18 Dec 2021, Double Blind Randomized Controlled Trial, Iran, peer-reviewed, mean age 66.4, 5 authors, study period 20 April, 2020 - 20 June, 2020, trial IRCT20200406046963N2.
Contact: dr.hazrati.e@ajaums.ac.ir, dr.hazrati.e@gmail.com.
A randomized clinical trial study on the efficacy and safety of adalimumab and methylprednisolone pulse therapy in the treatment of COVID-19 patients with acute respiratory distress syndrome
Immunopathologia Persa, doi:10.34172/ipp.2022.30322
Introduction: Adalimumab reduces the expression of the angiotensin-converting enzyme (ACE2) receptor at the cell surface, therefore it is thought to be effective in treating patients with COVID-19. Objectives: The present study was conducted to evaluate the effectiveness of adalimumab and pulsed corticosteroids in treating patients with severe acute respiratory failure due to COVID-19. Patients and Methods: The present double-blind clinical trial study was carried out on patients with COVID-19 referred to Imam Reza hospital, Tehran. Patients were randomly divided into two groups of intervention (patients under standard treatment according to the national protocol of Iran + methylprednisolone + adalimumab) and control (patients under standard treatment according to the national protocol of Iran + methylprednisolone). Results: The patients' hospitalization information shows that the duration of patients' hospitalization in the intervention group was significantly shorter than their counterparts in the control group (P = 0.041). Serum levels of total bilirubin on the ninth day (P = 0.043) and GCS (Glasgow coma scale) on the ninth day (P = 0.041) and tenth (P = 0.039) in the adalimumab group were significantly increased compared to the control group. However, the direct bilirubin value on the eighth day (P=0.031), serum creatinine on the 8th (P = 0.047), 9th (P = 0.047) and 10th (P = 0.047) days and also PEEF (pericarditis/pericardial effusion) on the tenth day were significantly lower in the intervention group than the control group.
Conclusion: The administration of adalimumab significantly increases the GCS of COVID-19 patients and reduces the length of hospital stay. Trial Registration: This study is designed as a double-blind clinical trial (identifier: IRCT20200406046963N2, https://www.irct.ir/trial/55011 ), and has been approved by the ethics committee in biomedical research of AJA University of Medical Sciences (#IR.AJAUMS.REC.1400.
Authors' contribution
Conflicts of interest The authors declare there is no conflict of interest.
Ethical issues The present study was conducted according to the Declaration of Helsinki and with the approval of the ethics committee of AJA University of Medical Sciences. Thus, the Ethics Committee in Biomedical Research of AJA University of Medical Sciences reviewed the implementation process of this study and declared it applicable following its approved protocols (IR.AJAUMS. REC.1400.032). According to the structure defined for the study (randomized clinical trial), informed consent was obtained from all participants before the intervention, and the whole process of treatment and intervention was free in the form of research. The trial protocol was approved by the Iranian Clinical Trial Registry (identifier: IRCT20200406046963N2, https://www.irct. ir/trial/55011 ). Besides, ethical issues (including plagiarism, data fabrication, double publication) have been completely observed by the authors.
Funding/Support The present study was conducted with the financial and moral support of the Vice-Chancellor for Research and Technology of AJA University of Medical Sciences (Grant#97001405).
References
References
Bertoncelli, Guidarini, Greca, Ratti, Falcinella et al., COVID19: potential cardiovascular issues in pediatric patients, Acta Biomed, doi:10.23750/abm.v91i2.9655
Bertuzzo, Mari, Pasetto, Miccoli, Casagrandi et al., The geography of COVID-19 spread in Italy and implications for the relaxation of confinement measures, Nat Commun, doi:10.1038/s41467-020-18050-2
Chathukulam, Tharamangalam, The Kerala model in the time of COVID19: Rethinking state, society and democracy, World Dev, doi:10.1016/j.worlddev.2020.105207
Clark, Vagenas, Panesar, Cope, What does tumour necrosis factor excess do to the immune system long term?, Ann Rheum Dis, doi:10.1136/ard.2005.042523
Conti, Lasagni, Bigi, Pellacani, Evolution of COVID-19 infection in four psoriatic patients treated with biological drugs, J Eur Acad Dermatol Venereol, doi:10.1111/jdv.16587
Devaux, Rolain, Colson, Raoult, New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19?, Int J Antimicrob Agents, doi:10.1016/j.ijantimicag.2020.105938
Ferrannini, Barbieri, Biggeri, Tommaso, Industrial policy for sustainable human development in the post-Covid19 era, World Dev, doi:10.1016/j.worlddev.2020.105215
Gatto, Bertuzzo, Mari, Miccoli, Carraro et al., Spread and dynamics of the COVID-19 epidemic in Italy: Effects of emergency containment measures, Proc Natl Acad Sci, doi:10.1073/pnas.2004978117
Ghormade, Kumar, Tingne, Keoliya, Distribution & diagnostic efficacy of cardiac markers CK-MB & LDH in pericardial fluid for postmortem diagnosis of ischemic heart disease, J Forensic Leg Med, doi:10.1016/j.jflm.2014.09.011
Huang, Gnanasegaran, Bomanji, COVID19 -Nuclear Medicine Departments, be prepared!, Nucl Med Commun, doi:10.1097/MNM.0000000000001183
Huang, Wang, Li, Ren, Zhao et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet, doi:10.1016/S0140-6736(20)30183-5
Jaffe, Landt, Parvin, Abendschein, Geltman et al., Comparative sensitivity of cardiac troponin I and lactate dehydrogenase isoenzymes for diagnosing acute myocardial infarction, Clin Chem, doi:10.1093/clinchem/42.11.1770
Kabbani, Olds, Does COVID19 Infect the Brain? If So, Smokers Might Be at a Higher Risk, Mol Pharmacol, doi:10.1124/molpharm.120.000014
Kenny, Mallon, COVID19-clinical presentation and therapeutic considerations, Biochem Biophys Res Commun, doi:10.1016/j.bbrc.2020.11.021
Kuppen, Jonges, Van De Velde, Vahrmeijer, Tollenaar et al., Liver and tumour tissue concentrations of TNF-alpha in cancer patients treated with TNF-alpha and melphalan by isolated liver perfusion, Br J Cancer, doi:10.1038/bjc.1997.255
Mair, Cardiac troponin I and troponin T: are enzymes still relevant as cardiac markers?, Clin Chim Acta, doi:10.1016/s0009-8981(96)06436-4
Mandel, Harari, Gurevich, Achiron, Cytokine prediction of mortality in COVID19 patients, Cytokine, doi:10.1016/j.cyto.2020.155190
Martins, Li, Baskin, Jialal, Keffer, Comparison of cardiac troponin I and lactate dehydrogenase isoenzymes for the late diagnosis of myocardial injury, Am J Clin Pathol, doi:10.1093/ajcp/106.6.705
Ratliff, Estes, Myles, Shirey, Mcmahon, Diagnosis of chloroquine cardiomyopathy by endomyocardial biopsy, N Engl J Med, doi:10.1056/NEJM198701223160405
Soy, Keser, Atagündüz, Tabak, Atagündüz et al., Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment, Clin Rheumatol, doi:10.1007/s10067-020-05190-5
Thalha, Lee, Besari, Omar, Have we found the panacea to COVID-19 with remdesivir, an old but newly packaged drug?, J R Coll Physicians Edinb, doi:10.4997/JRCPE.2020.217
Thienemann, Pinto, Grobbee, Boehm, Bazargani et al., World Heart Federation Briefing on Prevention: Coronavirus Disease 2019 (COVID-19) in Low-Income Countries, Glob Heart, doi:10.5334/gh.778
Vafaie, State-of-the-art diagnosis of myocardial infarction, Diagnosis (Berl), doi:10.1515/dx-2016-0024
Zuckier, Gordon, COVID-19 in the Nuclear Medicine Department, be prepared for ventilation scans as well!, Nucl Med Commun, doi:10.1097/MNM.0000000000001196
DOI record:
{
"DOI": "10.34172/ipp.2022.30322",
"ISSN": [
"2423-8015"
],
"URL": "http://dx.doi.org/10.34172/ipp.2022.30322",
"abstract": "<jats:p>Introduction: Adalimumab reduces the expression of the angiotensin-converting enzyme (ACE2) receptor at the cell surface, therefore it is thought to be effective in treating patients with COVID-19. Objectives: The present study was conducted to evaluate the effectiveness of adalimumab and pulsed corticosteroids in treating patients with severe acute respiratory failure due to COVID-19. Patients and Methods: The present double-blind clinical trial study was carried out on patients with COVID-19 referred to Imam Reza hospital, Tehran. Patients were randomly divided into two groups of intervention (patients under standard treatment according to the national protocol of Iran + methylprednisolone + adalimumab) and control (patients under standard treatment according to the national protocol of Iran + methylprednisolone). Results: The patients’ hospitalization information shows that the duration of patients’ hospitalization in the intervention group was significantly shorter than their counterparts in the control group (P=0.041). Serum levels of total bilirubin on the ninth day (P=0.043) and GCS (Glasgow coma scale) on the ninth day (P=0.041) and tenth (P=0.039) in the adalimumab group were significantly increased compared to the control group. However, the direct bilirubin value on the eighth day (P=0.031), serum creatinine on the 8th (P=0.047), 9th (P=0.047) and 10th (P=0.047) days and also PEEF (pericarditis/pericardial effusion) on the tenth day were significantly lower in the intervention group than the control group. Conclusion: The administration of adalimumab significantly increases the GCS of COVID-19 patients and reduces the length of hospital stay. Trial Registration: This study is designed as a double-blind clinical trial (identifier: IRCT20200406046963N2, https://www.irct.ir/trial/55011), and has been approved by the ethics committee in biomedical research of AJA University of Medical Sciences (#IR.AJAUMS.REC.1400.032).</jats:p>",
"assertion": [
{
"label": "Journal Owner",
"name": "journal_owner",
"value": "Nickan Research Institute"
},
{
"label": "Journal Publisher",
"name": "journal_publisher",
"value": "Nickan Research Institute"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "2021-11-02"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "2021-12-04"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 3,
"value": "2021-12-18"
}
],
"author": [
{
"ORCID": "https://orcid.org/0000-0002-0798-3308",
"affiliation": [
{
"name": "Department of Medical, School of Medicine, AJA University of Medical Sciences, Tehran, Iran"
}
],
"authenticated-orcid": true,
"family": "Yazdani",
"given": "Omid",
"sequence": "first"
},
{
"ORCID": "https://orcid.org/0000-0002-3213-0948",
"affiliation": [
{
"name": "Department of Infectious Diseases, School of Medicine, AJA University of Medical Sciences, Tehran, Iran"
}
],
"authenticated-orcid": true,
"family": "Hamidi Farahani",
"given": "Ramin",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-1757-4361",
"affiliation": [
{
"name": "Faculty Medicine, AJA University of Medical Sciences, Tehran, Iran"
}
],
"authenticated-orcid": true,
"family": "Mosaed",
"given": "Reza",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Faculty of Aerospace and Subaquatic Medicine, AJA University of Medical Sciences, Tehran, Iran"
}
],
"family": "Nezami Asl",
"given": "Amir",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-6987-7404",
"affiliation": [
{
"name": "Department of Anesthesiology and Critical Care, AJA University of Medical Sciences, Tehran, Iran"
},
{
"name": "Trauma and Surgery Research Center, AJA University of Medical Sciences, Tehran, Iran"
}
],
"authenticated-orcid": true,
"family": "Hazrati",
"given": "Ebrahim",
"sequence": "additional"
}
],
"container-title": "Immunopathologia Persa",
"container-title-short": "Immunopathol Persa",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"immunopathol.com"
]
},
"created": {
"date-parts": [
[
2022,
5,
30
]
],
"date-time": "2022-05-30T07:09:47Z",
"timestamp": 1653894587000
},
"deposited": {
"date-parts": [
[
2023,
6,
20
]
],
"date-time": "2023-06-20T08:10:52Z",
"timestamp": 1687248652000
},
"indexed": {
"date-parts": [
[
2025,
2,
21
]
],
"date-time": "2025-02-21T23:23:42Z",
"timestamp": 1740180222726,
"version": "3.37.3"
},
"is-referenced-by-count": 0,
"issue": "2",
"issued": {
"date-parts": [
[
2021,
12,
18
]
]
},
"journal-issue": {
"issue": "2",
"published-online": {
"date-parts": [
[
2023,
6,
17
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://immunopathol.com/PDF/ipp-9-30322.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://immunopathol.com/PDF/ipp-9-30322.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "20123",
"original-title": [],
"page": "30322",
"prefix": "10.34172",
"published": {
"date-parts": [
[
2021,
12,
18
]
]
},
"published-online": {
"date-parts": [
[
2021,
12,
18
]
]
},
"publisher": "Maad Rayan Publishing Company",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://immunopathol.com/Article/ipp-30322"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "A randomized clinical trial study on the efficacy and safety of adalimumab and methylprednisolone pulse therapy in the treatment of COVID-19 patients with acute respiratory distress syndrome",
"type": "journal-article",
"update-policy": "https://doi.org/10.34172/crossmark_policy",
"volume": "9"
}