COVID-19 Rebound After VV116 vs Nirmatrelvir-Ritonavir Treatment
et al., JAMA Network Open, doi:10.1001/jamanetworkopen.2024.1765, ChiCTR2200066811, Mar 2024
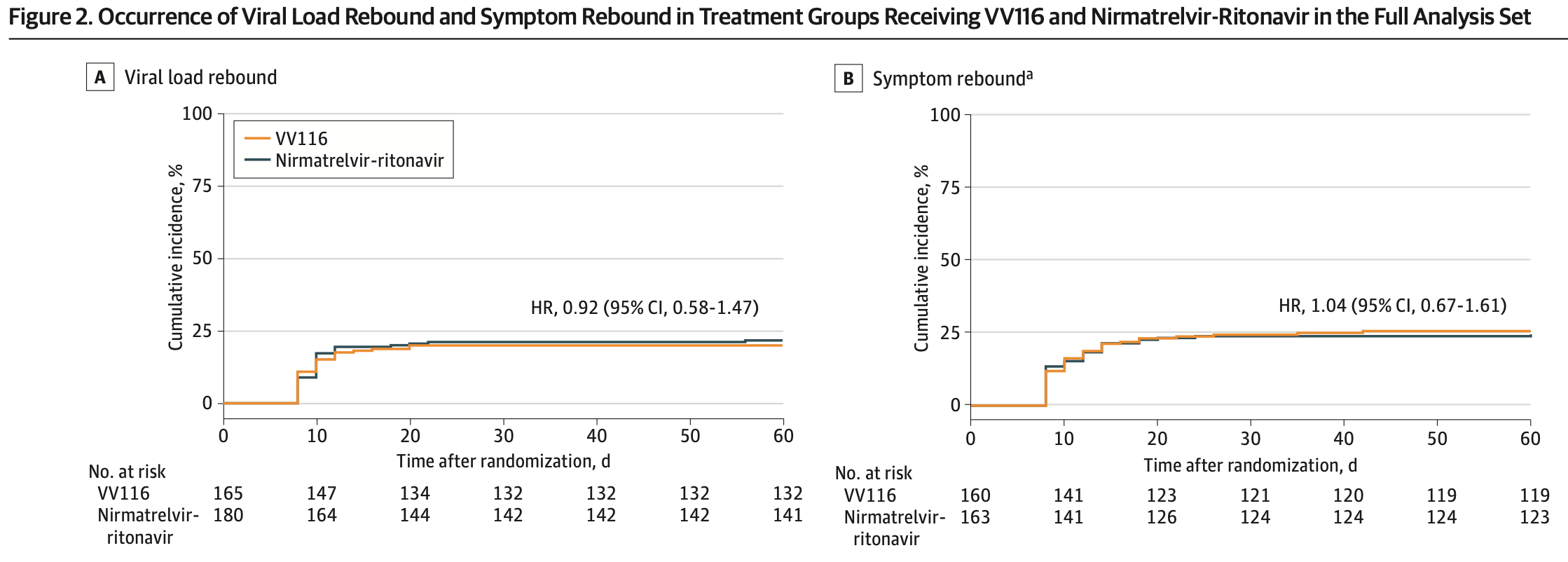
RCT showing high rates of viral and symptom rebound with both paxlovid and deuremidevir (VV116).
There are multiple potential reasons, with one being the highly specific targets within viral replication (Mpro and RdRp respectively). Paxlovid and deuremidevir may limit viral replication, however they may not eliminate all virions or prevent the virus from persisting in a latent or low-replicative state within some cells. Replication may resume after drug levels drop leading to rebound.
Combination therapy using additional treatments with different mechanisms of action may be more effective.
Study covers paxlovid and deuremidevir.
Yang et al., 13 Mar 2024, Randomized Controlled Trial, China, peer-reviewed, 31 authors, study period 20 December, 2022 - 19 January, 2023, trial ChiCTR2200066811.
COVID-19 Rebound After VV116 vs Nirmatrelvir-Ritonavir Treatment
JAMA Network Open, doi:10.1001/jamanetworkopen.2024.1765
IMPORTANCE With the widespread use of anti-SARS-CoV-2 drugs, accumulating data have revealed potential viral load rebound after treatment. OBJECTIVE To compare COVID-19 rebound after a standard 5-day course of antiviral treatment with VV116 vs nirmatrelvir-ritonavir. DESIGN, SETTING, AND PARTICIPANTS This is a single-center, investigator-blinded, randomized clinical trial conducted in Shanghai, China. Adult patients with mild-to-moderate COVID-19 and within 5 days of SARS-CoV-2 infection were enrolled between December 20, 2022, and January 19, 2023, and randomly allocated to receive either VV116 or nirmatrelvir-ritonavir. INTERVENTIONS Participants in the VV116 treatment group received oral 600-mg VV116 tablets every 12 hours on day 1 and 300 mg every 12 hours on days 2 through 5. Participants in the nirmatrelvir-ritonavir treatment group received oral nirmatrelvir-ritonavir tablets with 300 mg of nirmatrelvir plus 100 mg of ritonavir every 12 hours for 5 days. Participants were followed up every other day until day 28 and every week until day 60.
MAIN OUTCOMES AND MEASURES The primary outcome was viral load rebound (VLR), defined as a half-log increase in viral RNA copies per milliliter compared with treatment completion. Secondary outcomes included a reduction in the cycle threshold value of 1.5 or more, time until VLR, and symptom rebound, defined as an increase of more than 2 points in symptom score compared with treatment completion. The primary outcome and secondary outcomes were analyzed using the full analysis set. Sensitivity analyses were conducted using the per protocol set. Adverse events were analyzed using the safety analysis set.
RESULTS The full analysis set included 345 participants (mean [SD] age, 53.2 [16.8] years; 175 [50.7%] were men) who received VV116 (n = 165) or nirmatrelvir-ritonavir (n = 180). Viral load rebound occurred in 33 patients (20.0%) in the VV116 group and 39 patients (21.7%) in the nirmatrelvir-ritonavir group (P = .70). Symptom rebound occurred in 41 of 160 patients (25.6%) in the VV116 group and 40 of 163 patients (24.5%) in the nirmatrelvir-ritonavir group (P = .82). Viral whole-genome sequencing of 24 rebound cases revealed the same lineage at baseline and at viral load rebound in each case. CONCLUSIONS AND RELEVANCE In this randomized clinical trial of patients with mild-to-moderate COVID-19, viral load rebound and symptom rebound were both common after a standard 5-day (continued) Key Points Question How common is COVID-19 rebound after a standard 5-day course of treatment with VV116 vs nirmatrelvirritonavir? Findings In this randomized clinical trial of 345 patients with mild-to-moderate COVID-19, viral load rebound occurred in 20.0% of patients in the VV116 group and 21.7% of patients in the nirmatrelvirritonavir group. Symptom rebound occurred in 25.6% of patients in the VV116 group and 24.5% of patients in the nirmatrelvir-ritonavir group. Meaning Viral load rebound and symptom rebound are both..
References
Anderson, Caubel, Rusnak, EPIC-HR Trial Investigators. Nirmatrelvir-ritonavir and viral load rebound in COVID-19, N Engl J Med, doi:10.1056/NEJMc2205944
Cao, Gao, Bao, VV116 versus nirmatrelvir-ritonavir for oral treatment of COVID-19, N Engl J Med, doi:10.1056/NEJMoa2208822
Carlin, Clark, Chaillon, Virologic and immunologic characterization of coronavirus disease 2019 recrudescence after nirmatrelvir/ritonavir treatment, Clin Infect Dis, doi:10.1093/cid/ciac496
Charness, Gupta, Stack, Rebound of SARS-CoV-2 infection after nirmatrelvir-ritonavir treatment, N Engl J Med, doi:10.1056/NEJMc2206449
Deo, Choudhary, Moser, ACTIV-2/A5401 Study Team. Symptom and viral rebound in untreated SARS-CoV-2 infection, Ann Intern Med, doi:10.7326/M22-2381
Edelstein, Boucau, Uddin, SARS-CoV-2 virologic rebound with nirmatrelvir-ritonavir therapy: an observational study, Ann Intern Med, doi:10.7326/M23-1756
Fajnzylber, Regan, Coxen, Massachusetts Consortium for Pathogen Readiness. SARS-CoV-2 viral load is associated with increased disease severity and mortality, Nat Commun, doi:10.1038/s41467-020-19057-5
Hammond, Leister-Tebbe, Gardner, Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19, N Engl J Med, doi:10.1056/NEJMoa2118542
Menni, Valdes, Polidori, Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of Omicron and Delta variant dominance: a prospective observational study from the ZOE COVID Study, Lancet, doi:10.1016/S0140-6736(22)00327-0
Ranganath, Horo, Challener, Rebound phenomenon after nirmatrelvir/ritonavir treatment of coronavirus disease 2019 (COVID-19) in high-risk persons, Clin Infect Dis, doi:10.1093/cid/ciac481
Se, Biddle, Talbot, Symptoms, viral loads, and rebound among COVID-19 outpatients treated with nirmatrelvir/ritonavir compared to propensity score matched untreated individuals, Clin Infect Dis
Wang, Berger, Davis, Kaelber, Volkow et al., COVID-19 rebound after Paxlovid and molnupiravir during January-June 2022, doi:10.1101/2022.06.21.22276724
Wong, Lau, Au, Viral burden rebound in hospitalised patients with COVID-19 receiving oral antivirals in Hong Kong: a population-wide retrospective cohort study, Lancet Infect Dis, doi:10.1016/S1473-3099(22)00873-8
Xie, Yin, Zhang, JAMA Network Open | Infectious Diseases COVID-19 Rebound After VV116 vs Nirmatrelvir-Ritonavir Treatment, JAMA Network Open, doi:10.1038/s41422-021-00570-1
Zheng, Liu, Lu, Impact of national Omicron outbreak at the end of 2022 on the future outlook of COVID-19 in China, Emerg Microbes Infect, doi:10.1080/22221751.2023.2191738
DOI record:
{
"DOI": "10.1001/jamanetworkopen.2024.1765",
"ISSN": [
"2574-3805"
],
"URL": "http://dx.doi.org/10.1001/jamanetworkopen.2024.1765",
"abstract": "<jats:sec><jats:title>Importance</jats:title><jats:p>With the widespread use of anti–SARS-CoV-2 drugs, accumulating data have revealed potential viral load rebound after treatment.</jats:p></jats:sec><jats:sec><jats:title>Objective</jats:title><jats:p>To compare COVID-19 rebound after a standard 5-day course of antiviral treatment with VV116 vs nirmatrelvir-ritonavir.</jats:p></jats:sec><jats:sec><jats:title>Design, Setting, and Participants</jats:title><jats:p>This is a single-center, investigator-blinded, randomized clinical trial conducted in Shanghai, China. Adult patients with mild-to-moderate COVID-19 and within 5 days of SARS-CoV-2 infection were enrolled between December 20, 2022, and January 19, 2023, and randomly allocated to receive either VV116 or nirmatrelvir-ritonavir.</jats:p></jats:sec><jats:sec><jats:title>Interventions</jats:title><jats:p>Participants in the VV116 treatment group received oral 600-mg VV116 tablets every 12 hours on day 1 and 300 mg every 12 hours on days 2 through 5. Participants in the nirmatrelvir-ritonavir treatment group received oral nirmatrelvir-ritonavir tablets with 300 mg of nirmatrelvir plus 100 mg of ritonavir every 12 hours for 5 days. Participants were followed up every other day until day 28 and every week until day 60.</jats:p></jats:sec><jats:sec><jats:title>Main Outcomes and Measures</jats:title><jats:p>The primary outcome was viral load rebound (VLR), defined as a half-log increase in viral RNA copies per milliliter compared with treatment completion. Secondary outcomes included a reduction in the cycle threshold value of 1.5 or more, time until VLR, and symptom rebound, defined as an increase of more than 2 points in symptom score compared with treatment completion. The primary outcome and secondary outcomes were analyzed using the full analysis set. Sensitivity analyses were conducted using the per protocol set. Adverse events were analyzed using the safety analysis set.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>The full analysis set included 345 participants (mean [SD] age, 53.2 [16.8] years; 175 [50.7%] were men) who received VV116 (n = 165) or nirmatrelvir-ritonavir (n = 180). Viral load rebound occurred in 33 patients (20.0%) in the VV116 group and 39 patients (21.7%) in the nirmatrelvir-ritonavir group (<jats:italic>P</jats:italic> = .70). Symptom rebound occurred in 41 of 160 patients (25.6%) in the VV116 group and 40 of 163 patients (24.5%) in the nirmatrelvir-ritonavir group (<jats:italic>P</jats:italic> = .82). Viral whole-genome sequencing of 24 rebound cases revealed the same lineage at baseline and at viral load rebound in each case.</jats:p></jats:sec><jats:sec><jats:title>Conclusions and Relevance</jats:title><jats:p>In this randomized clinical trial of patients with mild-to-moderate COVID-19, viral load rebound and symptom rebound were both common after a standard 5-day course of treatment with either VV116 or nirmatrelvir-ritonavir. Prolongation of treatment duration might be investigated to reduce COVID-19 rebound.</jats:p></jats:sec><jats:sec><jats:title>Trial Registration</jats:title><jats:p>Chinese Clinical Trial Registry Identifier: <jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"uri\" xlink:href=\"https://www.chictr.org.cn/showprojEN.html?proj=188019\">ChiCTR2200066811</jats:ext-link></jats:p></jats:sec>",
"author": [
{
"affiliation": [
{
"name": "Emergency Department, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China"
}
],
"family": "Yang",
"given": "Zhitao",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine and Metabolic Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China"
},
{
"name": "National Clinical Research Center for Metabolic Diseases (Shanghai), Key Laboratory for Endocrine and Metabolic Diseases of the National Health Commission, National Research Center for Translational Medicine, State Key Laboratory of Medical Genomics, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China"
}
],
"family": "Xu",
"given": "Yu",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine and Metabolic Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China"
},
{
"name": "National Clinical Research Center for Metabolic Diseases (Shanghai), Key Laboratory for Endocrine and Metabolic Diseases of the National Health Commission, National Research Center for Translational Medicine, State Key Laboratory of Medical Genomics, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China"
}
],
"family": "Zheng",
"given": "Ruizhi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine and Metabolic Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China"
},
{
"name": "National Clinical Research Center for Metabolic Diseases (Shanghai), Key Laboratory for Endocrine and Metabolic Diseases of the National Health Commission, National Research Center for Translational Medicine, State Key Laboratory of Medical Genomics, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China"
}
],
"family": "Ye",
"given": "Lei",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Shanghai Institute of Hematology, National Research Center for Translational Medicine, State Key Laboratory of Medical Genomics, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China"
}
],
"family": "Lv",
"given": "Gang",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China"
}
],
"family": "Cao",
"given": "Zhujun",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine and Metabolic Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China"
},
{
"name": "National Clinical Research Center for Metabolic Diseases (Shanghai), Key Laboratory for Endocrine and Metabolic Diseases of the National Health Commission, National Research Center for Translational Medicine, State Key Laboratory of Medical Genomics, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China"
}
],
"family": "Han",
"given": "Rulai",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine and Metabolic Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China"
},
{
"name": "National Clinical Research Center for Metabolic Diseases (Shanghai), Key Laboratory for Endocrine and Metabolic Diseases of the National Health Commission, National Research Center for Translational Medicine, State Key Laboratory of Medical Genomics, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China"
}
],
"family": "Li",
"given": "Mian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Geriatrics, Medical Center on Aging, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China"
}
],
"family": "Zhu",
"given": "Yuanyue",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine and Metabolic Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China"
},
{
"name": "National Clinical Research Center for Metabolic Diseases (Shanghai), Key Laboratory for Endocrine and Metabolic Diseases of the National Health Commission, National Research Center for Translational Medicine, State Key Laboratory of Medical Genomics, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China"
}
],
"family": "Cao",
"given": "Qiuyu",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine and Metabolic Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China"
},
{
"name": "National Clinical Research Center for Metabolic Diseases (Shanghai), Key Laboratory for Endocrine and Metabolic Diseases of the National Health Commission, National Research Center for Translational Medicine, State Key Laboratory of Medical Genomics, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China"
}
],
"family": "Ding",
"given": "Yi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine and Metabolic Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China"
},
{
"name": "National Clinical Research Center for Metabolic Diseases (Shanghai), Key Laboratory for Endocrine and Metabolic Diseases of the National Health Commission, National Research Center for Translational Medicine, State Key Laboratory of Medical Genomics, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China"
}
],
"family": "Wang",
"given": "Jiqiu",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Shanghai Institute of Hematology, National Research Center for Translational Medicine, State Key Laboratory of Medical Genomics, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China"
}
],
"family": "Tan",
"given": "Yun",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Shanghai Institute of Hematology, National Research Center for Translational Medicine, State Key Laboratory of Medical Genomics, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China"
}
],
"family": "Liu",
"given": "Feng",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, Research Laboratory of Clinical Virology, National Research Center for Translational Medicine, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China"
}
],
"family": "Wei",
"given": "Dong",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, Research Laboratory of Clinical Virology, National Research Center for Translational Medicine, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China"
}
],
"family": "Tan",
"given": "Wei",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Research and Development Administration Department, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China"
}
],
"family": "Jiang",
"given": "Weiwei",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Gastroenterology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China"
}
],
"family": "Sun",
"given": "Jing",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine and Metabolic Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China"
},
{
"name": "National Clinical Research Center for Metabolic Diseases (Shanghai), Key Laboratory for Endocrine and Metabolic Diseases of the National Health Commission, National Research Center for Translational Medicine, State Key Laboratory of Medical Genomics, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China"
}
],
"family": "Sun",
"given": "Shouyue",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pediatrics, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China"
}
],
"family": "Shao",
"given": "Jie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of General Surgery, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China"
}
],
"family": "Deng",
"given": "Yang",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Medical Affairs, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China"
}
],
"family": "Gao",
"given": "Weiyi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine and Metabolic Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China"
},
{
"name": "National Clinical Research Center for Metabolic Diseases (Shanghai), Key Laboratory for Endocrine and Metabolic Diseases of the National Health Commission, National Research Center for Translational Medicine, State Key Laboratory of Medical Genomics, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China"
}
],
"family": "Wang",
"given": "Weiqing",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of General Surgery, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China"
}
],
"family": "Zhao",
"given": "Ren",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Administrative Office, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China"
}
],
"family": "Qiu",
"given": "Liping",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Emergency Department, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China"
}
],
"family": "Chen",
"given": "Erzhen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, Research Laboratory of Clinical Virology, National Research Center for Translational Medicine, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China"
},
{
"name": "Clinical Trials Center, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China"
}
],
"family": "Zhang",
"given": "Xinxin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Shanghai Institute of Hematology, National Research Center for Translational Medicine, State Key Laboratory of Medical Genomics, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China"
}
],
"family": "Wang",
"given": "Shengyue",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine and Metabolic Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China"
},
{
"name": "National Clinical Research Center for Metabolic Diseases (Shanghai), Key Laboratory for Endocrine and Metabolic Diseases of the National Health Commission, National Research Center for Translational Medicine, State Key Laboratory of Medical Genomics, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China"
}
],
"family": "Ning",
"given": "Guang",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Trials Center, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China"
}
],
"family": "Xu",
"given": "Yiping",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine and Metabolic Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China"
},
{
"name": "National Clinical Research Center for Metabolic Diseases (Shanghai), Key Laboratory for Endocrine and Metabolic Diseases of the National Health Commission, National Research Center for Translational Medicine, State Key Laboratory of Medical Genomics, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China"
}
],
"family": "Bi",
"given": "Yufang",
"sequence": "additional"
}
],
"container-title": "JAMA Network Open",
"container-title-short": "JAMA Netw Open",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
3,
13
]
],
"date-time": "2024-03-13T15:18:39Z",
"timestamp": 1710343119000
},
"deposited": {
"date-parts": [
[
2024,
3,
13
]
],
"date-time": "2024-03-13T15:18:46Z",
"timestamp": 1710343126000
},
"indexed": {
"date-parts": [
[
2024,
3,
14
]
],
"date-time": "2024-03-14T00:53:16Z",
"timestamp": 1710377596136
},
"is-referenced-by-count": 0,
"issue": "3",
"issued": {
"date-parts": [
[
2024,
3,
13
]
]
},
"journal-issue": {
"issue": "3",
"published-print": {
"date-parts": [
[
2024,
3,
4
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://jamanetwork.com/journals/jamanetworkopen/articlepdf/2816033/yang_2024_oi_240089_1709228357.67437.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "10",
"original-title": [],
"page": "e241765",
"prefix": "10.1001",
"published": {
"date-parts": [
[
2024,
3,
13
]
]
},
"published-online": {
"date-parts": [
[
2024,
3,
13
]
]
},
"publisher": "American Medical Association (AMA)",
"reference": [
{
"DOI": "10.1056/NEJMc2206449",
"article-title": "Rebound of SARS-CoV-2 infection after nirmatrelvir-ritonavir treatment.",
"author": "Charness",
"doi-asserted-by": "publisher",
"first-page": "1045",
"issue": "11",
"journal-title": "N Engl J Med",
"key": "zoi240089r1",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1093/cid/ciac496",
"article-title": "Virologic and immunologic characterization of coronavirus disease 2019 recrudescence after nirmatrelvir/ritonavir treatment.",
"author": "Carlin",
"doi-asserted-by": "publisher",
"first-page": "e530",
"issue": "3",
"journal-title": "Clin Infect Dis",
"key": "zoi240089r2",
"volume": "76",
"year": "2023"
},
{
"DOI": "10.1093/cid/ciac481",
"article-title": "Rebound phenomenon after nirmatrelvir/ritonavir treatment of coronavirus disease 2019 (COVID-19) in high-risk persons.",
"author": "Ranganath",
"doi-asserted-by": "publisher",
"first-page": "e537",
"issue": "3",
"journal-title": "Clin Infect Dis",
"key": "zoi240089r4",
"volume": "76",
"year": "2023"
},
{
"DOI": "10.1016/S1473-3099(22)00873-8",
"article-title": "Viral burden rebound in hospitalised patients with COVID-19 receiving oral antivirals in Hong Kong: a population-wide retrospective cohort study.",
"author": "Wong",
"doi-asserted-by": "publisher",
"first-page": "683",
"issue": "6",
"journal-title": "Lancet Infect Dis",
"key": "zoi240089r6",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.1056/NEJMc2205944",
"article-title": "Nirmatrelvir-ritonavir and viral load rebound in COVID-19.",
"author": "Anderson",
"doi-asserted-by": "publisher",
"first-page": "1047",
"issue": "11",
"journal-title": "N Engl J Med",
"key": "zoi240089r7",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2208822",
"article-title": "VV116 versus nirmatrelvir-ritonavir for oral treatment of COVID-19.",
"author": "Cao",
"doi-asserted-by": "publisher",
"first-page": "406",
"issue": "5",
"journal-title": "N Engl J Med",
"key": "zoi240089r8",
"volume": "388",
"year": "2023"
},
{
"DOI": "10.1016/S0140-6736(22)00327-0",
"article-title": "Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of Omicron and Delta variant dominance: a prospective observational study from the ZOE COVID Study.",
"author": "Menni",
"doi-asserted-by": "publisher",
"first-page": "1618",
"issue": "10335",
"journal-title": "Lancet",
"key": "zoi240089r9",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.1080/22221751.2023.2191738",
"article-title": "Impact of national Omicron outbreak at the end of 2022 on the future outlook of COVID-19 in China.",
"author": "Zheng",
"doi-asserted-by": "publisher",
"issue": "1",
"journal-title": "Emerg Microbes Infect",
"key": "zoi240089r10",
"volume": "12",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19.",
"author": "Hammond",
"doi-asserted-by": "publisher",
"first-page": "1397",
"issue": "15",
"journal-title": "N Engl J Med",
"key": "zoi240089r11",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.7326/M22-2381",
"article-title": "Symptom and viral rebound in untreated SARS-CoV-2 infection.",
"author": "Deo",
"doi-asserted-by": "publisher",
"first-page": "348",
"issue": "3",
"journal-title": "Ann Intern Med",
"key": "zoi240089r12",
"volume": "176",
"year": "2023"
},
{
"article-title": "Symptoms, viral loads, and rebound among COVID-19 outpatients treated with nirmatrelvir/ritonavir compared to propensity score matched untreated individuals.",
"author": "Smith-Jeffcoat",
"journal-title": "Clin Infect Dis",
"key": "zoi240089r13"
},
{
"DOI": "10.7326/M23-1756",
"article-title": "SARS-CoV-2 virologic rebound with nirmatrelvir-ritonavir therapy: an observational study.",
"author": "Edelstein",
"doi-asserted-by": "publisher",
"first-page": "1577",
"issue": "12",
"journal-title": "Ann Intern Med",
"key": "zoi240089r14",
"volume": "176",
"year": "2023"
},
{
"DOI": "10.1038/s41467-020-19057-5",
"article-title": "SARS-CoV-2 viral load is associated with increased disease severity and mortality.",
"author": "Fajnzylber",
"doi-asserted-by": "publisher",
"first-page": "5493",
"issue": "1",
"journal-title": "Nat Commun",
"key": "zoi240089r15",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1038/s41422-021-00570-1",
"article-title": "Design and development of an oral remdesivir derivative VV116 against SARS-CoV-2.",
"author": "Xie",
"doi-asserted-by": "publisher",
"first-page": "1212",
"issue": "11",
"journal-title": "Cell Res",
"key": "zoi240089r16",
"volume": "31",
"year": "2021"
},
{
"DOI": "10.1056/NEJMc2202092",
"article-title": "Duration of shedding of culturable virus in SARS-CoV-2 Omicron (BA.1) infection.",
"author": "Boucau",
"doi-asserted-by": "publisher",
"first-page": "275",
"issue": "3",
"journal-title": "N Engl J Med",
"key": "zoi240089r17",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1017/ice.2022.124",
"article-title": "Similar duration of viral shedding of the severe acute respiratory coronavirus virus 2 (SARS-CoV-2) Delta variant between vaccinated and incompletely vaccinated individuals.",
"author": "Kandel",
"doi-asserted-by": "publisher",
"first-page": "1002",
"issue": "6",
"journal-title": "Infect Control Hosp Epidemiol",
"key": "zoi240089r18",
"volume": "44",
"year": "2023"
},
{
"DOI": "10.1093/cid/ciac663",
"article-title": "Clinical, virologic, and immunologic evaluation of symptomatic coronavirus disease 2019 rebound following nirmatrelvir/ritonavir treatment.",
"author": "Epling",
"doi-asserted-by": "publisher",
"first-page": "573",
"issue": "4",
"journal-title": "Clin Infect Dis",
"key": "zoi240089r19",
"volume": "76",
"year": "2023"
},
{
"key": "zoi240089r3",
"unstructured": "US Centers for Disease Control and Prevention. CDC Health Advisory: COVID-19 rebound after Paxlovid treatment. May 24, 2022. Accessed August 21, 2023. https://emergency.cdc.gov/han/2022/pdf/CDC_HAN_467.pdf"
},
{
"DOI": "10.1101/2022.06.21.22276724",
"doi-asserted-by": "crossref",
"key": "zoi240089r5",
"unstructured": "Wang? L, Berger? NA, Davis? PB, Kaelber? DC, Volkow? ND, Xu? R. COVID-19 rebound after Paxlovid and molnupiravir during January-June 2022. medRxiv. Preprint posted online June 22, 2022. doi:10.1101/2022.06.21.22276724"
}
],
"reference-count": 19,
"references-count": 19,
"relation": {},
"resource": {
"primary": {
"URL": "https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2816033"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [
"A Randomized Clinical Trial"
],
"title": "COVID-19 Rebound After VV116 vs Nirmatrelvir-Ritonavir Treatment",
"type": "journal-article",
"volume": "7"
}