Clinical outcomes, virological efficacy and safety of nitazoxanide in the treatment of patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials
et al., Expert Review of Anti-infective Therapy, doi:10.1080/14787210.2022.2142117, Nov 2022
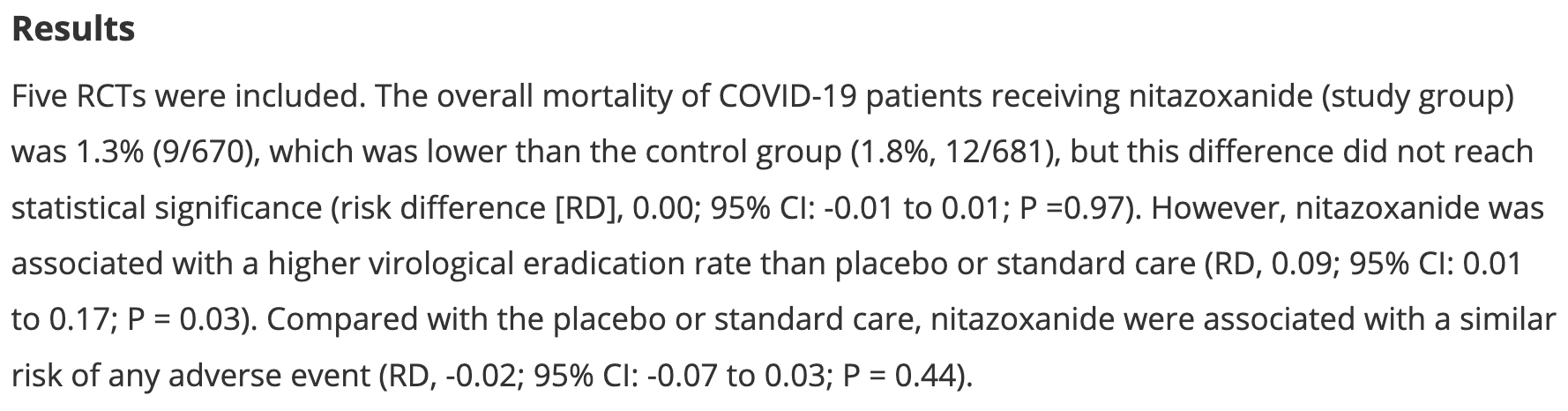
Meta analysis of 5 RCTs showing improved viral clearance with nitazoxanide. Mortality was lower, but without statistical significance.
2 meta-analyses show significant improvements with nitazoxanide for oxygen therapy1 and
viral clearance1,2.
Currently there are 14 nitazoxanide for COVID-19 studies, showing 42% lower mortality [-24‑73%], 82% lower ventilation [24‑96%], 28% lower ICU admission [-21‑57%], 61% lower hospitalization [22‑80%], and 13% fewer cases [-31‑42%].
Weng et al., 3 Nov 2022, Randomized Controlled Trial, peer-reviewed, 5 authors.
DOI record:
{
"DOI": "10.1080/14787210.2022.2142117",
"ISSN": [
"1478-7210",
"1744-8336"
],
"URL": "http://dx.doi.org/10.1080/14787210.2022.2142117",
"alternative-id": [
"10.1080/14787210.2022.2142117"
],
"assertion": [
{
"label": "Peer Review Statement",
"name": "peerreview_statement",
"order": 1,
"value": "The publishing and review policy for this title is described in its Aims & Scope."
},
{
"URL": "http://www.tandfonline.com/action/journalInformation?show=aimsScope&journalCode=ierz20",
"label": "Aim & Scope",
"name": "aims_and_scope_url",
"order": 2,
"value": "http://www.tandfonline.com/action/journalInformation?show=aimsScope&journalCode=ierz20"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2022-09-21"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "2022-10-25"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 3,
"value": "2022-11-03"
}
],
"author": [
{
"affiliation": [
{
"name": "Division of Hospital Medicine, Department of Internal Medicine, Chi Mei Medical Center, Tainan, Taiwan"
}
],
"family": "Weng",
"given": "Tzu-Chieh",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Pharmacy, Chi Mei Medical Center, Liouying, Taiwan"
}
],
"family": "Weng",
"given": "Teng-Song",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6334-2388",
"affiliation": [
{
"name": "Division of Hospital Medicine, Department of Internal Medicine, Chi Mei Medical Center, Tainan, Taiwan"
}
],
"authenticated-orcid": false,
"family": "Lai",
"given": "Chih-Cheng",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Intensive Care Medicine, Chi Mei Medical Center, Liouying, Taiwan"
}
],
"family": "Chao",
"given": "Chien-Ming",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, Division of Infection Disease, Kaohsiung Veterans General Hospital, Tainan Branch, Tainan, Taiwan"
},
{
"name": "Department of Health Care Administration, Chang Jung Christian University, Tainan, Taiwan"
},
{
"name": "Department of Food Nutrition, Chung-Hwa University of Medical Technology, Tainan, Taiwan"
}
],
"family": "Wang",
"given": "Jui-Hsiang",
"sequence": "additional"
}
],
"container-title": "Expert Review of Anti-infective Therapy",
"container-title-short": "Expert Review of Anti-infective Therapy",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"www.tandfonline.com"
]
},
"created": {
"date-parts": [
[
2022,
11,
1
]
],
"date-time": "2022-11-01T11:28:49Z",
"timestamp": 1667302129000
},
"deposited": {
"date-parts": [
[
2023,
1,
3
]
],
"date-time": "2023-01-03T11:47:40Z",
"timestamp": 1672746460000
},
"indexed": {
"date-parts": [
[
2023,
12,
2
]
],
"date-time": "2023-12-02T14:33:33Z",
"timestamp": 1701527613112
},
"is-referenced-by-count": 1,
"issue": "12",
"issued": {
"date-parts": [
[
2022,
11,
3
]
]
},
"journal-issue": {
"issue": "12",
"published-print": {
"date-parts": [
[
2022,
12,
2
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://www.tandfonline.com/doi/pdf/10.1080/14787210.2022.2142117",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "301",
"original-title": [],
"page": "1615-1622",
"prefix": "10.1080",
"published": {
"date-parts": [
[
2022,
11,
3
]
]
},
"published-online": {
"date-parts": [
[
2022,
11,
3
]
]
},
"published-print": {
"date-parts": [
[
2022,
12,
2
]
]
},
"publisher": "Informa UK Limited",
"reference": [
{
"DOI": "10.1016/j.ijantimicag.2020.105924",
"doi-asserted-by": "publisher",
"key": "cit0001"
},
{
"key": "cit0002",
"unstructured": "World Health Organization. Accessed on 2022 Oct 24. https://covid19.who.int/."
},
{
"DOI": "10.1080/14760584.2021.1949293",
"doi-asserted-by": "publisher",
"key": "cit0003"
},
{
"DOI": "10.2991/jegh.k.201028.001",
"doi-asserted-by": "publisher",
"key": "cit0004"
},
{
"DOI": "10.3390/v14081706",
"doi-asserted-by": "publisher",
"key": "cit0005"
},
{
"DOI": "10.1056/NEJMoa2118542",
"doi-asserted-by": "publisher",
"key": "cit0006"
},
{
"DOI": "10.1056/NEJMoa2116044",
"doi-asserted-by": "publisher",
"key": "cit0007"
},
{
"DOI": "10.1056/NEJMoa2116846",
"doi-asserted-by": "publisher",
"key": "cit0008"
},
{
"DOI": "10.1093/jac/dkab093",
"doi-asserted-by": "publisher",
"key": "cit0009"
},
{
"key": "cit0010",
"unstructured": "National Institutes of Health. https://www.covid19treatmentguidelines.nih.gov/ Accessed on 2022 Oct 24)."
},
{
"DOI": "10.1016/j.ijantimicag.2022.106545",
"doi-asserted-by": "publisher",
"key": "cit0011"
},
{
"DOI": "10.1016/j.jmii.2021.05.011",
"doi-asserted-by": "publisher",
"key": "cit0012"
},
{
"DOI": "10.1080/17512433.2022.2078701",
"doi-asserted-by": "publisher",
"key": "cit0013"
},
{
"DOI": "10.1074/jbc.M109.029470",
"doi-asserted-by": "publisher",
"key": "cit0014"
},
{
"DOI": "10.1016/S1473-3099(14)70717-0",
"doi-asserted-by": "publisher",
"key": "cit0015"
},
{
"DOI": "10.1021/acsinfecdis.0c00478",
"doi-asserted-by": "publisher",
"key": "cit0016"
},
{
"DOI": "10.1016/j.jiph.2016.04.001",
"doi-asserted-by": "publisher",
"key": "cit0017"
},
{
"DOI": "10.1038/s41598-018-28172-9",
"doi-asserted-by": "publisher",
"key": "cit0018"
},
{
"DOI": "10.1016/j.isci.2019.07.003",
"doi-asserted-by": "publisher",
"key": "cit0019"
},
{
"DOI": "10.1152/ajplung.00170.2020",
"doi-asserted-by": "publisher",
"key": "cit0020"
},
{
"DOI": "10.1016/j.ymthe.2020.12.016",
"doi-asserted-by": "publisher",
"key": "cit0021"
},
{
"author": "Lian E",
"journal-title": "bioRxiv",
"key": "cit0022"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"doi-asserted-by": "publisher",
"key": "cit0023"
},
{
"DOI": "10.1183/13993003.03725-2020",
"doi-asserted-by": "publisher",
"key": "cit0024"
},
{
"DOI": "10.3389/fmed.2022.844728",
"doi-asserted-by": "publisher",
"key": "cit0025"
},
{
"DOI": "10.1016/j.eclinm.2022.101310",
"doi-asserted-by": "publisher",
"key": "cit0026"
},
{
"DOI": "10.1016/j.eclinm.2021.100981",
"doi-asserted-by": "publisher",
"key": "cit0027"
},
{
"DOI": "10.1016/j.ajg.2022.04.005",
"doi-asserted-by": "publisher",
"key": "cit0028"
},
{
"DOI": "10.1016/j.jclinepi.2021.02.003",
"doi-asserted-by": "publisher",
"key": "cit0029"
},
{
"DOI": "10.1136/bmj.l4898",
"doi-asserted-by": "publisher",
"key": "cit0030"
},
{
"DOI": "10.1007/s00228-022-03380-5",
"doi-asserted-by": "publisher",
"key": "cit0031"
},
{
"DOI": "10.1101/2021.03.03.21252509",
"doi-asserted-by": "publisher",
"key": "cit0032"
},
{
"DOI": "10.1002/cpt.2463",
"doi-asserted-by": "publisher",
"key": "cit0033"
}
],
"reference-count": 33,
"references-count": 33,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.tandfonline.com/doi/full/10.1080/14787210.2022.2142117"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Virology",
"Infectious Diseases",
"Microbiology (medical)",
"Microbiology"
],
"subtitle": [],
"title": "Clinical outcomes, virological efficacy and safety of nitazoxanide in the treatment of patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1080/tandf_crossmark_01",
"volume": "20"
}