The Effect of Nitazoxanide on the Clinical Outcomes in Patients with COVID-19: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
et al., Clinical Drug Investigation, doi:10.1007/s40261-022-01213-y, Oct 2022
Systematic review and meta analysis of 6 RCTs, showing significantly improved viral clearance and lower oxygen requirements with nitazoxanide, but no significant differences for mortality, ICU admission, and recovery.
2 meta-analyses show significant improvements with nitazoxanide for oxygen therapy1 and
viral clearance1,2.
Currently there are 14 nitazoxanide for COVID-19 studies, showing 42% lower mortality [-24‑73%], 82% lower ventilation [24‑96%], 28% lower ICU admission [-21‑57%], 61% lower hospitalization [22‑80%], and 13% fewer cases [-31‑42%].
Abuelazm et al., 31 Oct 2022, peer-reviewed, 7 authors.
Contact: dr.mabuelazm@gmail.com.
The Effect of Nitazoxanide on the Clinical Outcomes in Patients with COVID-19: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Clinical Drug Investigation, doi:10.1007/s40261-022-01213-y
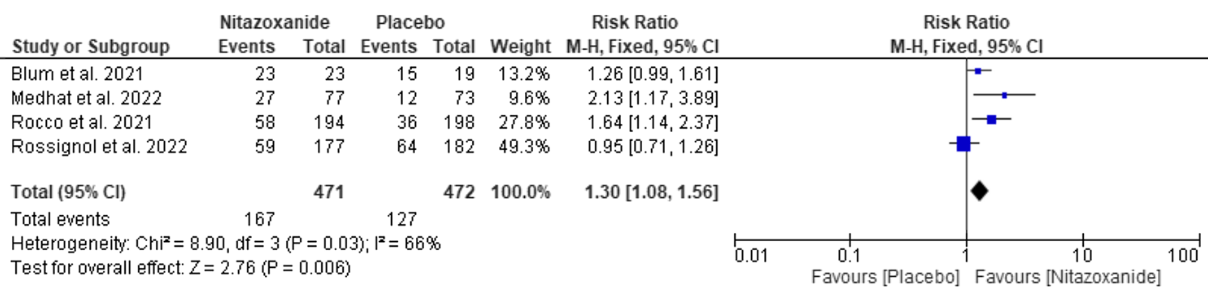
Background and Objective Nitazoxanide, a US Food and Drug Administration-approved antiparasitic agent, was reported to be effective in treating coronavirus disease 2019 . The lack of effective and precise treatments for COVID-19 infection earlier in the pandemic forced us to depend on symptomatic, empirical, and supportive therapy, which overburdened intensive care units and exhausted hospital resources. Therefore, the aim of this systematic review and meta-analysis was to assess the efficacy and safety of nitazoxanide for COVID-19 treatment. Methods A systematic review and meta-analysis synthesizing relevant randomized controlled trials from six databases (MedRxiv, WOS, SCOPUS, EMBASE, PubMed, and CENTRAL) until 17 May 2022 was conducted. Risk ratio (RR) for dichotomous outcomes was used and data with a 95% confidence interval (CI) are presented. The protocol was registered in PROSPERO with ID: CRD42022334658. Results Six randomized controlled trials with 1412 patients were included in the analysis. Nitazoxanide was effective in accelerating viral clearance compared with placebo (RR: 1.30 with 95% CI 1.08, 1.56, p = 0.006) and reducing oxygen requirements (RR: 0.48 with 95% CI 0.39, 0.59, p = 0.00001), but we found no difference between nitazoxanide and placebo in improving clinical resolution (RR: 1.01 with 95% CI 0.94, 1.08, p = 0.88), reducing the mortality rate (RR: 0.88 with 95% CI 0.4, 1.91, p = 0.74), and intensive care unit admission (RR: 0.69 with 95% CI 0.43, 1.13, p = 0.14). Moreover, nitazoxanide was as safe as placebo (RR: 0.9 with 95% CI 0.72, 1.12, p = 0.34). Conclusions Compared with placebo, nitazoxanide was effective in expediting viral clearance and decreasing oxygen requirements. However, there was no difference between nitazoxanide and placebo regarding clinical response, all-cause mortality, and intensive care unit admission. Therefore, more large-scale studies are still needed to ascertain the clinical applicability of nitazoxanide in COVID-19.
Key Points Nitazoxanide is potentially effective in accelerating coronavirus disease 2019 (COVID-19) viral clearance and reducing oxygen requirements compared with placebo.
Supplementary Information The online version contains supplementary material available at https:// doi. org/ 10. 1007/ s40261-022-01213-y.
Declarations Funding No funding was received for the preparation of this article.
Conflicts of Interest/Competing Interests Mohamed Abuelazm, Ahmed Ghanem, Ahmed K. Awad, Ramadan Abdelmoez Farahat, Fatma Labieb, Basant E. Katamesh, and Basel Abdelazeem have no conflicts of interest that are directly relevant to the content of this article.
Ethical Approval Not applicable. Consent to Participate Not applicable.
Consent for Publication Not applicable. Authors' Contributions MA conceived the idea. BA and MA designed the research workflow. BA and MA searched the databases. FL, RF, and BK screened the retrieved records, and MA resolved the conflicts. AK, FL, RF, and BK extracted relevant data, assessed the quality of evidence, and MA resolved the conflicts. MA and BA performed the analysis. MA and AG wrote the final manuscript. All authors have read and agreed to the final version of the manuscript. Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any
References
Akbari, Tabrizi, Lankarani, Aria, Vakili et al., The role of cytokine profile and lymphocyte subsets in the severity of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis, Life Sci
Al-Kuraishy, Brain and peripheral neuronal injury in Covid-19: the panorama and dispute, Appl Med Res, doi:10.5455/amr.20211025
Ashiru, Howe, Butters, Nitazoxanide, an antiviral thiazolide, depletes ATP-sensitive intracellular Ca(2+) stores, Virology
Attallah, El-Kadem, Negm, Elekhnawy, El-Masry et al., Promising antiviral activity of Agrimonia pilosa phytochemicals against severe acute respiratory syndrome coronavirus 2 supported with in vivo mice study, Pharmaceuticals
Baggiolini, Walz, Kunkel, Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils, J Clin Invest
Belardo, Cenciarelli, Frazia, Rossignol, Santoro, Synergistic effect of nitazoxanide with neuraminidase inhibitors against influenza A viruses in vitro, Antimicrob Agents Chemother
Bello-Perez, Sola, Novoa, Klionsky, Falco, Canonical and noncanonical autophagy as potential targets for COVID-19, Cells
Bernal, Da Silva, Musungaie, Kovalchuk, Gonzalez et al., Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients, N Engl J Med
Blum, Cimerman, Hunter, Tierno, Lacerda et al., Nitazoxanide superiority to placebo to treat moderate COVID-19: a pilot prove of concept randomized double-blind clinical trial, EClinicalMedicine
Bobrowski, Chen, Eastman, Itkin, Shinn et al., Synergistic and antagonistic drug combinations against SARS-CoV-2, Mol Ther
Cascella, Rajnik, Aleem, Dulebohn, Napoli, Features, evaluation, and treatment of coronavirus (COVID-19)
Chang, Mo, Yuan, Tao, Peng et al., Time kinetics of viral clearance and resolution of symptoms in novel coronavirus infection, Am J Respir Crit Care Med
Chen, Zheng, Liu, Yan, Xu et al., Plasma CRP level is positively associated with the severity of COVID-19, Ann Clin Microbiol Antimicrob
Dang, Xu, Ma, Chen, Yin et al., Nitazoxanide inhibits human norovirus replication and synergizes with ribavirin by activation of cellular antiviral response, Antimicrob Agents Chemother
Darif, Hammi, Kihel, Idrissi, Guessous et al., The pro-inflammatory cytokines in COVID-19 pathogenesis: what goes wrong?, Microb Pathog
Egger, Smith, Schneider, Minder, Bias in meta-analysis detected by a simple, graphical test, BMJ
Elalfy, Besheer, El-Mesery, El-Gilany, Soliman et al., Effect of a combination of nitazoxanide, ribavirin, and ivermectin plus zinc supplement (MANS. NRIZ study) on the clearance of mild COVID-19, J Med Virol
Feldmann, Maini, Woody, Holgate, Winter et al., Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed, Lancet
Freeman, Swartz, Targeting the NLRP3 inflammasome in severe COVID-19, Front Immunol
Guyatt, Oxman, Kunz, Vist, Falck-Ytter et al., What is "quality of evidence" and why is it important to clinicians?, BMJ
Guyatt, Oxman, Vist, Kunz, Falck-Ytter et al., GRADE: an emerging consensus on rating quality of evidence and strength of recommendations, BMJ
Hadjadj, Yatim, Barnabei, Corneau, Boussier et al., Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients, Science
Haffizulla, Hartman, Hoppers, Resnick, Samudrala et al., Effect of nitazoxanide in adults and adolescents with acute uncomplicated influenza: a double-blind, randomised, placebo-controlled, phase 2b/3 trial, Lancet Infect Dis
Han, Ma, Li, Liu, Zhao et al., Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors, Emerg Microbes Infect
Hickson, Margineantu, Hockenbery, Simon, Geballe, Inhibition of vaccinia virus replication by nitazoxanide, Virology
Higgins, Altman, Gøtzsche, Jüni, Moher et al., The Cochrane Collaboration's tool for assessing risk of bias in randomised trials, BMJ
Hm, Gareeb, Elekhnawy, Batiha, Nitazoxanide and COVID-19: a review, Mol Biol Rep, doi:10.1007/s11033-022-07822-2
Hong, Kim, Song, Choi, Lee et al., Nitazoxanide suppresses IL-6 production in LPS-stimulated mouse macrophages and TG-injected mice, Int Immunopharmacol
Huang, Chen, Yang, Guan, Liu et al., SARS-CoV-2 viral load in clinical samples from critically ill patients, Am J Respir Crit Care Med
Huang, Wang, Li, Ren, Zhao et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet
Jasenosky, Cadena, Mire, Borisevich, Haridas et al., The FDA-approved oral drug nitazoxanide amplifies host antiviral responses and inhibits Ebola virus, iScience
Kelleni, NSAIDs/nitazoxanide/azithromycin repurposed for COVID-19: potential mitigation of the cytokine storm interleukin-6 amplifier via immunomodulatory effects, Expert Rev Anti Infect Ther
Kim, Read, Fauci, Therapy for early COVID-19: a critical need, J Am Med Assoc
Lian, Mcalister, Ramirez, Chernoff, Went et al., Triple combination nitazoxanide, ribavirin, and hydroxychloroquine results in the multiplicative reduction of in vitro SARS-CoV-2 viral replication
Lin, Liu, Tang, He, The disease severity and clinical outcomes of the SARS-CoV-2 variants of concern, Front Public Health
Liu, Yang, Liao, Lin, Huang, Risk factors for COVID-19 progression and mortality in hospitalized patients without pre-existing comorbidities, J Infect Public Health
Lokhande, Devarajan, A review on possible mechanistic insights of nitazoxanide for repurposing in COVID-19, Eur J Pharmacol
Mahase, Covid-19: Pfizer's paxlovid is 89% effective in patients at risk of serious illness, company reports, BMJ
Mccullough, Alexander, Armstrong, Arvinte, Bain et al., Multifaceted highly targeted sequential multidrug treatment of early ambulatory high-risk SARS-CoV-2 infection (COVID-19), Rev Cardiovasc Med
Medhat, El-Kassas, Karam-Allah, Shafie, Abd-Elsalam et al., Sofosbuvir/ledipasvir in combination or nitazoxanide alone are safe and efficient treatments for COVID-19 infection: a randomized controlled trial for repurposing antivirals, Arab J Gastroenterol
Naveca, Nascimento, Souza, Corado, Nascimento et al., COVID-19 epidemic in the Brazilian state of Amazonas was driven by long-term persistence of endemic SARS-CoV-2 lineages and the recent emergence of the new variant of concern P.1, Res Sq, doi:10.21203/rs.3.rs-275494/v1
Nguyen, Zhang, Wang, Anang, Wang et al., Spike glycoprotein and host cell determinants of SARS-CoV-2 entry and cytopathic effects, J Virol
Padmanabhan, Padmanabhan, The "devil is in the dosing": targeting the interferon pathway by repositioning Nitazoxanide against COVID-19
Page, Mckenzie, Bossuyt, Boutron, Hoffmann et al., The PRISMA 2020 statement: an updated guideline for reporting systematic reviews, BMJ
Pietrocola, Bravo-San, Pedro, Targeting autophagy to counteract obesity-associated oxidative stress, Antioxidants
Procter, Ross, Pickard, Smith, Hanson et al., Clinical outcomes after early ambulatory multidrug therapy for high-risk SARS-CoV-2 (COVID-19) infection, Rev Cardiovasc Med
Qin, Zhou, Hu, Zhang, Yang et al., Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China Clin Infect Dis
Rajoli, Pertinez, Arshad, Box, Tatham et al., Dose prediction for repurposing nitazoxanide in SARS-CoV-2 treatment or chemoprophylaxis, Br J Clin Pharmacol
Rello, Storti, Belliato, Serrano, Clinical phenotypes of SARS-CoV-2: implications for clinicians and researchers, Eur Respir J
Risner, Tieu, Wang, Bakovic, Alem et al., Maraviroc inhibits SARS-CoV-2 multiplication and s-protein mediated cell fusion in cell culture, doi:10.1101/2020.08.12.246389
Rocco, Silva, Cruz, Melo, Tierno et al., Early use of nitazoxanide in mild COVID-19 disease: randomised, placebo-controlled trial, Eur Respir J
Rocco, Silva, Cruz, Tierno, Rabello et al., Nitazoxanide in patients hospitalized with COVID-19 pneumonia: a multicentre, randomized, double-blind, placebocontrolled trial, Front Med
Rossignol, Bardin, Fulgencio, Mogelnicki, Bréchot, A randomized double-blind placebo-controlled clinical trial of nitazoxanide for treatment of mild or moderate COVID-19, EClinicalMedicine
Rossignol, Bardin, Fulgencio, Mogelnicki, Bréchot, A randomized double-blind placebo-controlled clinical trial of nitazoxanide for treatment of mild or moderate COVID-19, eClinicalMedicine
Rossignol, Frazia, Chiappa, Ciucci, Santoro, Thiazolides, a new class of anti-influenza molecules targeting viral hemagglutinin at the post-translational level, J Biol Chem
Rossignol, Nitazoxanide, a new drug candidate for the treatment of Middle East respiratory syndrome coronavirus, J Infect Public Health
Rossignol, Nitazoxanide: a first-in-class broad-spectrum antiviral agent, Antiviral Res
Ruan, Yang, Wang, Jiang, Song, Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China Intensive Care Med
Shou, Kong, Wang, Tang, Wang et al., Tizoxanide inhibits inflammation in LPS-activated RAW264.7 macrophages via the suppression of NF-κB and MAPK activation, Inflammation
Silva, Espejo, Pereyra, Lynch, Thompson, Efficacy of nitazoxanide in reducing the viral load in COVID-19
Tang, Liu, Zhang, Xu, Wen, Cytokine storm in COVID-19: the current evidence and treatment strategies, Front Immunol
Valencia, Brief review on COVID-19: the 2020 pandemic caused by SARS-CoV-2, Cureus
Valle, Kim-Schulze, Huang, Beckmann, An inflammatory cytokine signature predicts COVID-19 severity and survival, Nat Med
Walker, Fitzgerald, Saunders, Lyon, Fisher et al., An open label, adaptive, phase 1 trial of high-dose oral nitazoxanide in healthy volunteers: an antiviral candidate for SARS-CoV-2, Clin Pharmacol Ther
Wu, Mcgoogan, Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention, J Am Med Assoc
Xu, Shi, Wang, Zhang, Huang et al., Pathological findings of COVID-19 associated with acute respiratory distress syndrome, Lancet Respir Med
Ye, Wang, Mao, The pathogenesis and treatment of the 'cytokine storm' in COVID-19, J Infect
Zhang, Guo, Lei, Liu, Wang et al., Frontline science: COVID-19 infection induces readily detectable morphologic and inflammation-related phenotypic changes in peripheral blood monocytes, J Leukoc Biol
Zhang, Hou, Ma, Li, Xue et al., The common risk factors for progression and mortality in COVID-19 patients: a meta-analysis, Arch Virol
Zheng, Yu, Feng, Lou, Zou, Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study, BMJ
Zhou, Yang, Zhou, Chen, Fang et al., A review of SARS-CoV2: compared with SARS-CoV and MERS-CoV, Front Med
DOI record:
{
"DOI": "10.1007/s40261-022-01213-y",
"ISSN": [
"1173-2563",
"1179-1918"
],
"URL": "http://dx.doi.org/10.1007/s40261-022-01213-y",
"alternative-id": [
"1213"
],
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "9 October 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 2,
"value": "31 October 2022"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Funding",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "No funding was received for the preparation of this article."
},
{
"group": {
"label": "Conflicts of Interest/Competing Interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Mohamed Abuelazm, Ahmed Ghanem, Ahmed K. Awad, Ramadan Abdelmoez Farahat, Fatma Labieb, Basant E. Katamesh, and Basel Abdelazeem have no conflicts of interest that are directly relevant to the content of this article."
},
{
"group": {
"label": "Ethical Approval",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "Not applicable."
},
{
"group": {
"label": "Consent to Participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 5,
"value": "Not applicable."
},
{
"group": {
"label": "Consent for Publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 6,
"value": "Not applicable."
},
{
"group": {
"label": "Availability of Data and Material",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 7,
"value": "The data are available upon request from the corresponding author."
},
{
"group": {
"label": "Code Availability",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 8,
"value": "Not applicable."
},
{
"group": {
"label": "Authors’ Contributions",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 9,
"value": "MA conceived the idea. BA and MA designed the research workflow. BA and MA searched the databases. FL, RF, and BK screened the retrieved records, and MA resolved the conflicts. AK, FL, RF, and BK extracted relevant data, assessed the quality of evidence, and MA resolved the conflicts. MA and BA performed the analysis. MA and AG wrote the final manuscript. All authors have read and agreed to the final version of the manuscript."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-2514-0689",
"affiliation": [],
"authenticated-orcid": false,
"family": "Abuelazm",
"given": "Mohamed",
"sequence": "first"
},
{
"affiliation": [],
"family": "Ghanem",
"given": "Ahmed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Awad",
"given": "Ahmed K.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Farahat",
"given": "Ramadan Abdelmoez",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Labieb",
"given": "Fatma",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Katamesh",
"given": "Basant E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abdelazeem",
"given": "Basel",
"sequence": "additional"
}
],
"container-title": "Clinical Drug Investigation",
"container-title-short": "Clin Drug Investig",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2022,
10,
31
]
],
"date-time": "2022-10-31T10:02:55Z",
"timestamp": 1667210575000
},
"deposited": {
"date-parts": [
[
2022,
10,
31
]
],
"date-time": "2022-10-31T11:09:06Z",
"timestamp": 1667214546000
},
"funder": [
{
"DOI": "10.13039/501100007950",
"doi-asserted-by": "crossref",
"name": "Tanta University"
}
],
"indexed": {
"date-parts": [
[
2022,
11,
1
]
],
"date-time": "2022-11-01T04:42:16Z",
"timestamp": 1667277736148
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
10,
31
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
10,
31
]
],
"date-time": "2022-10-31T00:00:00Z",
"timestamp": 1667174400000
}
},
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
10,
31
]
],
"date-time": "2022-10-31T00:00:00Z",
"timestamp": 1667174400000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1007/s40261-022-01213-y.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1007/s40261-022-01213-y/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1007/s40261-022-01213-y.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1007",
"published": {
"date-parts": [
[
2022,
10,
31
]
]
},
"published-online": {
"date-parts": [
[
2022,
10,
31
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"author": "DN Valencia",
"journal-title": "Cureus.",
"key": "1213_CR1",
"unstructured": "Valencia DN. Brief review on COVID-19: the 2020 pandemic caused by SARS-CoV-2. Cureus. 2020;12: e7386.",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.2648",
"author": "Z Wu",
"doi-asserted-by": "publisher",
"first-page": "1239",
"journal-title": "J Am Med Assoc",
"key": "1213_CR2",
"unstructured": "Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. J Am Med Assoc. 2020;323:1239–42.",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"author": "C Huang",
"doi-asserted-by": "publisher",
"first-page": "497",
"journal-title": "Lancet",
"key": "1213_CR3",
"unstructured": "Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506.",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1183/13993003.01028-2020",
"author": "J Rello",
"doi-asserted-by": "publisher",
"first-page": "2001028",
"issue": "5",
"journal-title": "Eur Respir J",
"key": "1213_CR4",
"unstructured": "Rello J, Storti E, Belliato M, Serrano R. Clinical phenotypes of SARS-CoV-2: implications for clinicians and researchers. Eur Respir J. 2020;55(5):2001028.",
"volume": "55",
"year": "2020"
},
{
"key": "1213_CR5",
"unstructured": "Cascella M, Rajnik M, Aleem A, Dulebohn SC, Di Napoli R. Features, evaluation, and treatment of coronavirus (COVID-19). In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2022."
},
{
"DOI": "10.3389/fimmu.2020.01708",
"author": "Y Tang",
"doi-asserted-by": "publisher",
"first-page": "1708",
"journal-title": "Front Immunol",
"key": "1213_CR6",
"unstructured": "Tang Y, Liu J, Zhang D, Xu Z, Ji J, Wen C. Cytokine storm in COVID-19: the current evidence and treatment strategies. Front Immunol. 2020;11:1708.",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1016/j.jinf.2020.03.037",
"author": "Q Ye",
"doi-asserted-by": "publisher",
"first-page": "607",
"journal-title": "J Infect",
"key": "1213_CR7",
"unstructured": "Ye Q, Wang B, Mao J. The pathogenesis and treatment of the 'cytokine storm’ in COVID-19. J Infect. 2020;80:607–13.",
"volume": "80",
"year": "2020"
},
{
"DOI": "10.1007/s00705-021-05012-2",
"author": "L Zhang",
"doi-asserted-by": "publisher",
"first-page": "2071",
"journal-title": "Arch Virol",
"key": "1213_CR8",
"unstructured": "Zhang L, Hou J, Ma F-Z, Li J, Xue S, Xu Z-G. The common risk factors for progression and mortality in COVID-19 patients: a meta-analysis. Arch Virol. 2021;166:2071–87.",
"volume": "166",
"year": "2021"
},
{
"DOI": "10.1016/j.jiph.2021.11.012",
"author": "W Liu",
"doi-asserted-by": "publisher",
"first-page": "13",
"journal-title": "J Infect Public Health",
"key": "1213_CR9",
"unstructured": "Liu W, Yang C, Liao Y-G, Wan F, Lin L, Huang X, et al. Risk factors for COVID-19 progression and mortality in hospitalized patients without pre-existing comorbidities. J Infect Public Health. 2022;15:13–20.",
"volume": "15",
"year": "2022"
},
{
"DOI": "10.3389/fpubh.2021.775224",
"author": "L Lin",
"doi-asserted-by": "publisher",
"journal-title": "Front Public Health",
"key": "1213_CR10",
"unstructured": "Lin L, Liu Y, Tang X, He D. The disease severity and clinical outcomes of the SARS-CoV-2 variants of concern. Front Public Health. 2021;9: 775224.",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1164/rccm.202003-0572LE",
"author": "Y Huang",
"doi-asserted-by": "publisher",
"first-page": "1435",
"journal-title": "Am J Respir Crit Care Med",
"key": "1213_CR11",
"unstructured": "Huang Y, Chen S, Yang Z, Guan W, Liu D, Lin Z, et al. SARS-CoV-2 viral load in clinical samples from critically ill patients. Am J Respir Crit Care Med. 2020;201:1435–8.",
"volume": "201",
"year": "2020"
},
{
"DOI": "10.1136/bmj.m1443",
"author": "S Zheng",
"doi-asserted-by": "publisher",
"journal-title": "BMJ",
"key": "1213_CR12",
"unstructured": "Zheng S, Fan J, Yu F, Feng B, Lou B, Zou Q, et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ. 2020;369: m1443.",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2116044",
"author": "A Jayk Bernal",
"doi-asserted-by": "publisher",
"first-page": "509",
"journal-title": "N Engl J Med",
"key": "1213_CR13",
"unstructured": "Jayk Bernal A, Gomes da Silva MM, Musungaie DB, Kovalchuk E, Gonzalez A, Delos Reyes V, et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med. 2022;386:509–20.",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1136/bmj.n2713",
"author": "E Mahase",
"doi-asserted-by": "publisher",
"journal-title": "BMJ",
"key": "1213_CR14",
"unstructured": "Mahase E. Covid-19: Pfizer’s paxlovid is 89% effective in patients at risk of serious illness, company reports. BMJ. 2021;375: n2713.",
"volume": "375",
"year": "2021"
},
{
"DOI": "10.31083/j.rcm.2020.04.264",
"author": "PA McCullough",
"doi-asserted-by": "publisher",
"first-page": "517",
"journal-title": "Rev Cardiovasc Med",
"key": "1213_CR15",
"unstructured": "McCullough PA, Alexander PE, Armstrong R, Arvinte C, Bain AF, Bartlett RP, et al. Multifaceted highly targeted sequential multidrug treatment of early ambulatory high-risk SARS-CoV-2 infection (COVID-19). Rev Cardiovasc Med. 2020;21:517–30.",
"volume": "21",
"year": "2020"
},
{
"DOI": "10.31083/j.rcm.2020.04.260",
"author": "BC Procter",
"doi-asserted-by": "publisher",
"first-page": "611",
"journal-title": "Rev Cardiovasc Med",
"key": "1213_CR16",
"unstructured": "Procter BC, Ross C, Pickard V, Smith E, Hanson C, McCullough PA. Clinical outcomes after early ambulatory multidrug therapy for high-risk SARS-CoV-2 (COVID-19) infection. Rev Cardiovasc Med. 2020;21:611–4.",
"volume": "21",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.22813",
"author": "PS Kim",
"doi-asserted-by": "publisher",
"first-page": "2149",
"journal-title": "J Am Med Assoc",
"key": "1213_CR17",
"unstructured": "Kim PS, Read SW, Fauci AS. Therapy for early COVID-19: a critical need. J Am Med Assoc. 2020;324:2149–50.",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1016/j.intimp.2012.03.002",
"author": "SK Hong",
"doi-asserted-by": "publisher",
"first-page": "23",
"journal-title": "Int Immunopharmacol",
"key": "1213_CR18",
"unstructured": "Hong SK, Kim HJ, Song CS, Choi IS, Lee JB, Park SY. Nitazoxanide suppresses IL-6 production in LPS-stimulated mouse macrophages and TG-injected mice. Int Immunopharmacol. 2012;13:23–7.",
"volume": "13",
"year": "2012"
},
{
"DOI": "10.1007/s10753-019-00994-3",
"author": "J Shou",
"doi-asserted-by": "publisher",
"first-page": "1336",
"journal-title": "Inflammation",
"key": "1213_CR19",
"unstructured": "Shou J, Kong X, Wang X, Tang Y, Wang C, Wang M, et al. Tizoxanide inhibits inflammation in LPS-activated RAW264.7 macrophages via the suppression of NF-κB and MAPK activation. Inflammation. 2019;42:1336–49.",
"volume": "42",
"year": "2019"
},
{
"DOI": "10.1016/j.antiviral.2014.07.014",
"author": "J-F Rossignol",
"doi-asserted-by": "publisher",
"first-page": "94",
"journal-title": "Antiviral Res",
"key": "1213_CR20",
"unstructured": "Rossignol J-F. Nitazoxanide: a first-in-class broad-spectrum antiviral agent. Antiviral Res. 2014;110:94–103.",
"volume": "110",
"year": "2014"
},
{
"DOI": "10.1016/j.jiph.2016.04.001",
"author": "J-F Rossignol",
"doi-asserted-by": "publisher",
"first-page": "227",
"journal-title": "J Infect Public Health",
"key": "1213_CR21",
"unstructured": "Rossignol J-F. Nitazoxanide, a new drug candidate for the treatment of Middle East respiratory syndrome coronavirus. J Infect Public Health. 2016;9:227–30.",
"volume": "9",
"year": "2016"
},
{
"DOI": "10.1016/j.eclinm.2022.101310",
"author": "J-F Rossignol",
"doi-asserted-by": "publisher",
"journal-title": "EClinicalMedicine.",
"key": "1213_CR22",
"unstructured": "Rossignol J-F, Bardin MC, Fulgencio J, Mogelnicki D, Bréchot C. A randomized double-blind placebo-controlled clinical trial of nitazoxanide for treatment of mild or moderate COVID-19. EClinicalMedicine. 2022;45: 101310.",
"volume": "45",
"year": "2022"
},
{
"DOI": "10.1016/j.virol.2014.05.015",
"author": "O Ashiru",
"doi-asserted-by": "publisher",
"first-page": "135",
"journal-title": "Virology",
"key": "1213_CR23",
"unstructured": "Ashiru O, Howe JD, Butters TD. Nitazoxanide, an antiviral thiazolide, depletes ATP-sensitive intracellular Ca(2+) stores. Virology. 2014;462–463:135–48.",
"volume": "462–463",
"year": "2014"
},
{
"DOI": "10.1128/AAC.03947-14",
"author": "G Belardo",
"doi-asserted-by": "publisher",
"first-page": "1061",
"journal-title": "Antimicrob Agents Chemother",
"key": "1213_CR24",
"unstructured": "Belardo G, Cenciarelli O, La Frazia S, Rossignol JF, Santoro MG. Synergistic effect of nitazoxanide with neuraminidase inhibitors against influenza A viruses in vitro. Antimicrob Agents Chemother. 2015;59:1061–9.",
"volume": "59",
"year": "2015"
},
{
"DOI": "10.1016/S1473-3099(14)70717-0",
"author": "J Haffizulla",
"doi-asserted-by": "publisher",
"first-page": "609",
"journal-title": "Lancet Infect Dis",
"key": "1213_CR25",
"unstructured": "Haffizulla J, Hartman A, Hoppers M, Resnick H, Samudrala S, Ginocchio C, et al. Effect of nitazoxanide in adults and adolescents with acute uncomplicated influenza: a double-blind, randomised, placebo-controlled, phase 2b/3 trial. Lancet Infect Dis. 2014;14:609–18.",
"volume": "14",
"year": "2014"
},
{
"DOI": "10.1016/j.isci.2019.07.003",
"author": "LD Jasenosky",
"doi-asserted-by": "publisher",
"first-page": "1279",
"journal-title": "iScience.",
"key": "1213_CR26",
"unstructured": "Jasenosky LD, Cadena C, Mire CE, Borisevich V, Haridas V, Ranjbar S, et al. The FDA-approved oral drug nitazoxanide amplifies host antiviral responses and inhibits Ebola virus. iScience. 2019;19:1279–90.",
"volume": "19",
"year": "2019"
},
{
"DOI": "10.1016/j.eclinm.2021.100981",
"author": "VF Blum",
"doi-asserted-by": "publisher",
"journal-title": "EClinicalMedicine.",
"key": "1213_CR27",
"unstructured": "Blum VF, Cimerman S, Hunter JR, Tierno P, Lacerda A, Soeiro A, et al. Nitazoxanide superiority to placebo to treat moderate COVID-19: a pilot prove of concept randomized double-blind clinical trial. EClinicalMedicine. 2021;37: 100981.",
"volume": "37",
"year": "2021"
},
{
"DOI": "10.1016/j.ajg.2022.04.005",
"author": "MA Medhat",
"doi-asserted-by": "publisher",
"first-page": "165",
"issue": "3",
"journal-title": "Arab J Gastroenterol.",
"key": "1213_CR28",
"unstructured": "Medhat MA, El-Kassas M, Karam-Allah H, Al Shafie A, Abd-Elsalam S, Moustafa E, et al. Sofosbuvir/ledipasvir in combination or nitazoxanide alone are safe and efficient treatments for COVID-19 infection: a randomized controlled trial for repurposing antivirals. Arab J Gastroenterol. 2022;23(3):165–71.",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.3389/fmed.2022.844728",
"author": "PRM Rocco",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Front Med",
"key": "1213_CR29",
"unstructured": "Rocco PRM, Silva PL, Cruz FF, Tierno PFGMM, Rabello E, Junior JC, et al. Nitazoxanide in patients hospitalized with COVID-19 pneumonia: a multicentre, randomized, double-blind, placebo-controlled trial. Front Med. 2022;9:1–13.",
"volume": "9",
"year": "2022"
},
{
"DOI": "10.1183/13993003.03725-2020",
"author": "PRM Rocco",
"doi-asserted-by": "publisher",
"first-page": "2003725",
"issue": "1",
"journal-title": "Eur Respir J",
"key": "1213_CR30",
"unstructured": "Rocco PRM, Silva PL, Cruz FF, Melo MAC, Tierno PFGMM, Moura MA, et al. Early use of nitazoxanide in mild COVID-19 disease: randomised, placebo-controlled trial. Eur Respir J. 2021;58(1):2003725.",
"volume": "58",
"year": "2021"
},
{
"DOI": "10.1016/j.eclinm.2022.101310",
"author": "JF Rossignol",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "eClinicalMedicine.",
"key": "1213_CR31",
"unstructured": "Rossignol JF, Bardin MC, Fulgencio J, Mogelnicki D, Bréchot C. A randomized double-blind placebo-controlled clinical trial of nitazoxanide for treatment of mild or moderate COVID-19. eClinicalMedicine. 2022;45:1–10.",
"volume": "45",
"year": "2022"
},
{
"key": "1213_CR32",
"unstructured": "Silva AM, Espejo A, Pereyra ML, Lynch M, Thompson M. Efficacy of nitazoxanide in reducing the viral load in COVID-19. 2021; p. 1–17."
},
{
"DOI": "10.1136/bmj.n71",
"author": "MJ Page",
"doi-asserted-by": "publisher",
"journal-title": "BMJ",
"key": "1213_CR33",
"unstructured": "Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71.",
"volume": "372",
"year": "2021"
},
{
"DOI": "10.1002/9781119536604",
"doi-asserted-by": "crossref",
"key": "1213_CR34",
"unstructured": "Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane handbook for systematic reviews of interventions. 2nd Ed. Chichester (UK): John Wiley & Sons; 2019."
},
{
"key": "1213_CR35",
"unstructured": "Covidence systematic review software. Melbourne, Australia; http://www.covidence.org/. Accessed 19 Oct 2022."
},
{
"DOI": "10.1136/bmj.d5928",
"author": "JPT Higgins",
"doi-asserted-by": "publisher",
"journal-title": "BMJ",
"key": "1213_CR36",
"unstructured": "Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343: d5928.",
"volume": "343",
"year": "2011"
},
{
"DOI": "10.1136/bmj.39490.551019.BE",
"author": "GH Guyatt",
"doi-asserted-by": "publisher",
"first-page": "995",
"issue": "7651",
"journal-title": "BMJ",
"key": "1213_CR37",
"unstructured": "Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schünemann HJ, GRADE Working Group. What is “quality of evidence” and why is it important to clinicians? BMJ. 2008;336(7651):995–8.",
"volume": "336",
"year": "2008"
},
{
"DOI": "10.1136/bmj.39489.470347.AD",
"author": "GH Guyatt",
"doi-asserted-by": "publisher",
"first-page": "924",
"issue": "7650",
"journal-title": "BMJ",
"key": "1213_CR38",
"unstructured": "Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ, GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6.",
"volume": "336",
"year": "2008"
},
{
"key": "1213_CR39",
"unstructured": "RevMan | Cochrane Training. https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman. Accessed 3 Aug 2021."
},
{
"DOI": "10.1136/bmj.315.7109.629",
"author": "M Egger",
"doi-asserted-by": "publisher",
"first-page": "629",
"issue": "7109",
"journal-title": "BMJ",
"key": "1213_CR40",
"unstructured": "Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.",
"volume": "315",
"year": "1997"
},
{
"DOI": "10.1074/jbc.M109.029470",
"author": "JF Rossignol",
"doi-asserted-by": "publisher",
"first-page": "29798",
"journal-title": "J Biol Chem",
"key": "1213_CR41",
"unstructured": "Rossignol JF, La Frazia S, Chiappa L, Ciucci A, Santoro MG. Thiazolides, a new class of anti-influenza molecules targeting viral hemagglutinin at the post-translational level. J Biol Chem. 2009;284:29798–808.",
"volume": "284",
"year": "2009"
},
{
"DOI": "10.1172/JCI114265",
"author": "M Baggiolini",
"doi-asserted-by": "publisher",
"first-page": "1045",
"journal-title": "J Clin Invest",
"key": "1213_CR42",
"unstructured": "Baggiolini M, Walz A, Kunkel SL. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989;84:1045–9.",
"volume": "84",
"year": "1989"
},
{
"DOI": "10.1016/j.virol.2018.03.023",
"author": "SE Hickson",
"doi-asserted-by": "publisher",
"first-page": "398",
"journal-title": "Virology",
"key": "1213_CR43",
"unstructured": "Hickson SE, Margineantu D, Hockenbery DM, Simon JA, Geballe AP. Inhibition of vaccinia virus replication by nitazoxanide. Virology. 2018;518:398–405.",
"volume": "518",
"year": "2018"
},
{
"DOI": "10.3390/ph14121313",
"author": "NGM Attallah",
"doi-asserted-by": "publisher",
"first-page": "1313",
"issue": "12",
"journal-title": "Pharmaceuticals (Basel)",
"key": "1213_CR44",
"unstructured": "Attallah NGM, El-Kadem AH, Negm WA, Elekhnawy E, El-Masry TA, Elmongy EI, et al. Promising antiviral activity of Agrimonia pilosa phytochemicals against severe acute respiratory syndrome coronavirus 2 supported with in vivo mice study. Pharmaceuticals (Basel). 2021;14(12):1313.",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.3389/fmed.2021.628370",
"author": "H Zhou",
"doi-asserted-by": "publisher",
"issue": "8",
"journal-title": "Front Med (Lausanne).",
"key": "1213_CR45",
"unstructured": "Zhou H, Yang J, Zhou C, Chen B, Fang H, Chen S, Zhang X, Wang L, Zhang L. A review of SARS-CoV2: compared with SARS-CoV and MERS-CoV. Front Med (Lausanne). 2021;7(8): 628370.",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.1007/s11033-022-07822-2",
"doi-asserted-by": "publisher",
"key": "1213_CR46",
"unstructured": "Al-kuraishy HM, Al-Gareeb AI, Elekhnawy E, Batiha GES. Nitazoxanide and COVID-19: a review. Mol Biol Rep. 2022;49:11169–76. https://doi.org/10.1007/s11033-022-07822-2."
},
{
"DOI": "10.1128/AAC.00707-18",
"author": "W Dang",
"doi-asserted-by": "publisher",
"first-page": "e00707",
"issue": "11",
"journal-title": "Antimicrob Agents Chemother",
"key": "1213_CR47",
"unstructured": "Dang W, Xu L, Ma B, Chen S, Yin Y, Chang KO, et al. Nitazoxanide inhibits human norovirus replication and synergizes with ribavirin by activation of cellular antiviral response. Antimicrob Agents Chemother. 2018;62(11):e00707-e718.",
"volume": "62",
"year": "2018"
},
{
"DOI": "10.5455/amr.20211025",
"doi-asserted-by": "publisher",
"key": "1213_CR48",
"unstructured": "M Al-kuraishy H. Brain and peripheral neuronal injury in Covid-19: the panorama and dispute. Appl Med Res. 2021;8:1–3. https://doi.org/10.5455/amr.20211025."
},
{
"author": "HT Nguyen",
"first-page": "e02304",
"issue": "5",
"journal-title": "J Virol",
"key": "1213_CR49",
"unstructured": "Nguyen HT, Zhang S, Wang Q, Anang S, Wang J, Ding H, et al. Spike glycoprotein and host cell determinants of SARS-CoV-2 entry and cytopathic effects. J Virol. 2020;95(5):e02304-e2320.",
"volume": "95",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2020.01518",
"author": "TL Freeman",
"doi-asserted-by": "publisher",
"first-page": "1518",
"journal-title": "Front Immunol",
"key": "1213_CR50",
"unstructured": "Freeman TL, Swartz TH. Targeting the NLRP3 inflammasome in severe COVID-19. Front Immunol. 2020;11:1518.",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1126/science.abc6027",
"author": "J Hadjadj",
"doi-asserted-by": "publisher",
"first-page": "718",
"journal-title": "Science",
"key": "1213_CR51",
"unstructured": "Hadjadj J, Yatim N, Barnabei L, Corneau A, Boussier J, Smith N, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718–24.",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1016/j.micpath.2021.104799",
"author": "D Darif",
"doi-asserted-by": "publisher",
"journal-title": "Microb Pathog",
"key": "1213_CR52",
"unstructured": "Darif D, Hammi I, Kihel A, El Idrissi SI, Guessous F, Akarid K. The pro-inflammatory cytokines in COVID-19 pathogenesis: what goes wrong? Microb Pathog. 2021;153: 104799.",
"volume": "153",
"year": "2021"
},
{
"DOI": "10.1002/jmv.26880",
"author": "H Elalfy",
"doi-asserted-by": "publisher",
"first-page": "3176",
"journal-title": "J Med Virol",
"key": "1213_CR53",
"unstructured": "Elalfy H, Besheer T, El-Mesery A, El-Gilany AH, Soliman MAA, Alhawarey A, et al. Effect of a combination of nitazoxanide, ribavirin, and ivermectin plus zinc supplement (MANS.NRIZ study) on the clearance of mild COVID-19. J Med Virol. 2021;93:3176–83.",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.3390/cells9071619",
"author": "M Bello-Perez",
"doi-asserted-by": "publisher",
"first-page": "1619",
"issue": "7",
"journal-title": "Cells",
"key": "1213_CR54",
"unstructured": "Bello-Perez M, Sola I, Novoa B, Klionsky DJ, Falco A. Canonical and noncanonical autophagy as potential targets for COVID-19. Cells. 2020;9(7):1619.",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.3390/antiox10010102",
"author": "F Pietrocola",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Antioxidants",
"key": "1213_CR55",
"unstructured": "Pietrocola F, Bravo-San Pedro JM. Targeting autophagy to counteract obesity-associated oxidative stress. Antioxidants. 2021;10:1–14.",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1007/s00134-020-05991-x",
"author": "Q Ruan",
"doi-asserted-by": "publisher",
"first-page": "846",
"journal-title": "China. Intensive Care Med.",
"key": "1213_CR56",
"unstructured": "Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan. China Intensive Care Med. 2020;46:846–8.",
"volume": "46",
"year": "2020"
},
{
"DOI": "10.1186/s12941-020-00362-2",
"author": "W Chen",
"doi-asserted-by": "publisher",
"first-page": "18",
"issue": "1",
"journal-title": "Ann Clin Microbiol Antimicrob",
"key": "1213_CR57",
"unstructured": "Chen W, Zheng KI, Liu S, Yan Z, Xu C, Qiao Z. Plasma CRP level is positively associated with the severity of COVID-19. Ann Clin Microbiol Antimicrob. 2020;19(1):18.",
"volume": "19",
"year": "2020"
},
{
"DOI": "10.1080/22221751.2020.1770129",
"author": "H Han",
"doi-asserted-by": "publisher",
"first-page": "1123",
"journal-title": "Emerg Microbes Infect.",
"key": "1213_CR58",
"unstructured": "Han H, Ma Q, Li C, Liu R, Zhao L, Wang W, et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect. 2020;9:1123–30.",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1038/s41591-020-1051-9",
"author": "DM Del Valle",
"doi-asserted-by": "publisher",
"first-page": "1636",
"issue": "10",
"journal-title": "Nat Med",
"key": "1213_CR59",
"unstructured": "Del Valle DM, Kim-Schulze S, Huang HH, Beckmann ND, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26(10):1636–43.",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1016/j.lfs.2020.118167",
"author": "H Akbari",
"doi-asserted-by": "publisher",
"journal-title": "Life Sci",
"key": "1213_CR60",
"unstructured": "Akbari H, Tabrizi R, Lankarani KB, Aria H, Vakili S, Asadian F, et al. The role of cytokine profile and lymphocyte subsets in the severity of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Life Sci. 2020;258: 118167.",
"volume": "258",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30858-8",
"author": "M Feldmann",
"doi-asserted-by": "publisher",
"first-page": "1407",
"journal-title": "Lancet",
"key": "1213_CR61",
"unstructured": "Feldmann M, Maini RN, Woody JN, Holgate ST, Winter G, Rowland M, et al. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet. 2020;395:1407–9.",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1080/14787210.2021.1939683",
"author": "MT Kelleni",
"doi-asserted-by": "publisher",
"first-page": "17",
"journal-title": "Expert Rev Anti Infect Ther",
"key": "1213_CR62",
"unstructured": "Kelleni MT. NSAIDs/nitazoxanide/azithromycin repurposed for COVID-19: potential mitigation of the cytokine storm interleukin-6 amplifier via immunomodulatory effects. Expert Rev Anti Infect Ther. 2022;20:17–21.",
"volume": "20",
"year": "2022"
},
{
"DOI": "10.21203/rs.3.rs-275494/v1",
"doi-asserted-by": "publisher",
"key": "1213_CR63",
"unstructured": "Naveca F, Nascimento V, Souza V, Corado A, Nascimento F, Silva G, et al. COVID-19 epidemic in the Brazilian state of Amazonas was driven by long-term persistence of endemic SARS-CoV-2 lineages and the recent emergence of the new variant of concern P.1. Res Sq. 2021;27:1–21. https://doi.org/10.21203/rs.3.rs-275494/v1. Accessed 26 Oct 2022."
},
{
"DOI": "10.1101/2020.08.12.246389",
"author": "KH Risner",
"doi-asserted-by": "publisher",
"journal-title": "bioRxiv [Preprint]",
"key": "1213_CR64",
"unstructured": "Risner KH, Tieu KV, Wang Y, Bakovic A, Alem F, Bhalla N, et al. Maraviroc inhibits SARS-CoV-2 multiplication and s-protein mediated cell fusion in cell culture. bioRxiv [Preprint]. 2020. https://doi.org/10.1101/2020.08.12.246389.",
"year": "2020"
},
{
"key": "1213_CR65",
"unstructured": "Lian E, McAlister C, Ramirez G, Chernoff DN, Went G, Hoopes J, et al. Triple combination nitazoxanide, ribavirin, and hydroxychloroquine results in the multiplicative reduction of in vitro SARS-CoV-2 viral replication. bioRxiv. 2020;2020.11.25.399055. http://biorxiv.org/content/early/2020/11/26/2020.11.25.399055.abstract. Accessed 10 Oct 2022."
},
{
"DOI": "10.1016/j.ymthe.2020.12.016",
"author": "T Bobrowski",
"doi-asserted-by": "publisher",
"first-page": "873",
"journal-title": "Mol Ther",
"key": "1213_CR66",
"unstructured": "Bobrowski T, Chen L, Eastman RT, Itkin Z, Shinn P, Chen CZ, et al. Synergistic and antagonistic drug combinations against SARS-CoV-2. Mol Ther. 2021;29:873–85.",
"volume": "29",
"year": "2021"
},
{
"DOI": "10.1164/rccm.202003-0524LE",
"author": "D Chang",
"doi-asserted-by": "publisher",
"first-page": "1150",
"journal-title": "Am J Respir Crit Care Med",
"key": "1213_CR67",
"unstructured": "Chang D, Mo G, Yuan X, Tao Y, Peng X, Wang FS, et al. Time kinetics of viral clearance and resolution of symptoms in novel coronavirus infection. Am J Respir Crit Care Med. 2020;201:1150–2.",
"volume": "201",
"year": "2020"
},
{
"DOI": "10.1016/j.ejphar.2020.173748",
"author": "AS Lokhande",
"doi-asserted-by": "publisher",
"journal-title": "Eur J Pharmacol",
"key": "1213_CR68",
"unstructured": "Lokhande AS, Devarajan PV. A review on possible mechanistic insights of nitazoxanide for repurposing in COVID-19. Eur J Pharmacol. 2021;891: 173748.",
"volume": "891",
"year": "2021"
},
{
"DOI": "10.1111/bcp.14619",
"author": "RKR Rajoli",
"doi-asserted-by": "publisher",
"first-page": "2078",
"journal-title": "Br J Clin Pharmacol",
"key": "1213_CR69",
"unstructured": "Rajoli RKR, Pertinez H, Arshad U, Box H, Tatham L, Curley P, et al. Dose prediction for repurposing nitazoxanide in SARS-CoV-2 treatment or chemoprophylaxis. Br J Clin Pharmacol. 2021;87:2078–88.",
"volume": "87",
"year": "2021"
},
{
"key": "1213_CR70",
"unstructured": "Padmanabhan S, Padmanabhan K. The \"devil is in the dosing\": targeting the interferon pathway by repositioning Nitazoxanide against COVID-19. https://www.researchgate.net/profile/Srivatsan-Padmanabhan/publication/340902283_The_devil_is_in_the_dosing_-_targeting_the_interferon_pathway_by_repositioning_Nitazoxanide_against_COVID-19/links/602b2218299bf1cc26cb6617/The-devil-is-in-the-dosing-targeting-the-interferon-pathway-by-repositioning-Nitazoxanide-against-COVID-19.pdf. Accessed 10 Oct 2022."
},
{
"DOI": "10.1002/cpt.2463",
"author": "LE Walker",
"doi-asserted-by": "publisher",
"first-page": "585",
"journal-title": "Clin Pharmacol Ther",
"key": "1213_CR71",
"unstructured": "Walker LE, FitzGerald R, Saunders G, Lyon R, Fisher M, Martin K, et al. An open label, adaptive, phase 1 trial of high-dose oral nitazoxanide in healthy volunteers: an antiviral candidate for SARS-CoV-2. Clin Pharmacol Ther. 2022;111:585–94.",
"volume": "111",
"year": "2022"
},
{
"DOI": "10.1093/cid/ciaa248",
"author": "C Qin",
"doi-asserted-by": "publisher",
"first-page": "762",
"journal-title": "China. Clin Infect Dis.",
"key": "1213_CR72",
"unstructured": "Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan. China Clin Infect Dis. 2020;71:762–8.",
"volume": "71",
"year": "2020"
},
{
"DOI": "10.1016/S2213-2600(20)30076-X",
"author": "Z Xu",
"doi-asserted-by": "publisher",
"first-page": "420",
"journal-title": "Lancet Respir Med",
"key": "1213_CR73",
"unstructured": "Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–2.",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1002/JLB.4HI0720-470R",
"author": "D Zhang",
"doi-asserted-by": "publisher",
"first-page": "13",
"journal-title": "J Leukoc Biol",
"key": "1213_CR74",
"unstructured": "Zhang D, Guo R, Lei L, Liu H, Wang Y, Wang Y, et al. Frontline science: COVID-19 infection induces readily detectable morphologic and inflammation-related phenotypic changes in peripheral blood monocytes. J Leukoc Biol. 2021;109:13–22.",
"volume": "109",
"year": "2021"
}
],
"reference-count": 74,
"references-count": 74,
"relation": {},
"resource": {
"primary": {
"URL": "https://link.springer.com/10.1007/s40261-022-01213-y"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology (medical)",
"General Medicine"
],
"subtitle": [],
"title": "The Effect of Nitazoxanide on the Clinical Outcomes in Patients with COVID-19: A Systematic Review and Meta-Analysis of Randomized Controlled Trials",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy"
}