The safety signal detection and analysis of monoclonal antibodies against SARS-CoV-2 based on real-world evidence – the suitable selectivity for different populations
et al., European Review for Medical and Pharmacological Sciences, doi:10.26355/eurrev_202404_35925, Apr 2024
42nd treatment shown to reduce risk in
May 2022, now with p = 0.0066 from 19 studies, recognized in 33 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
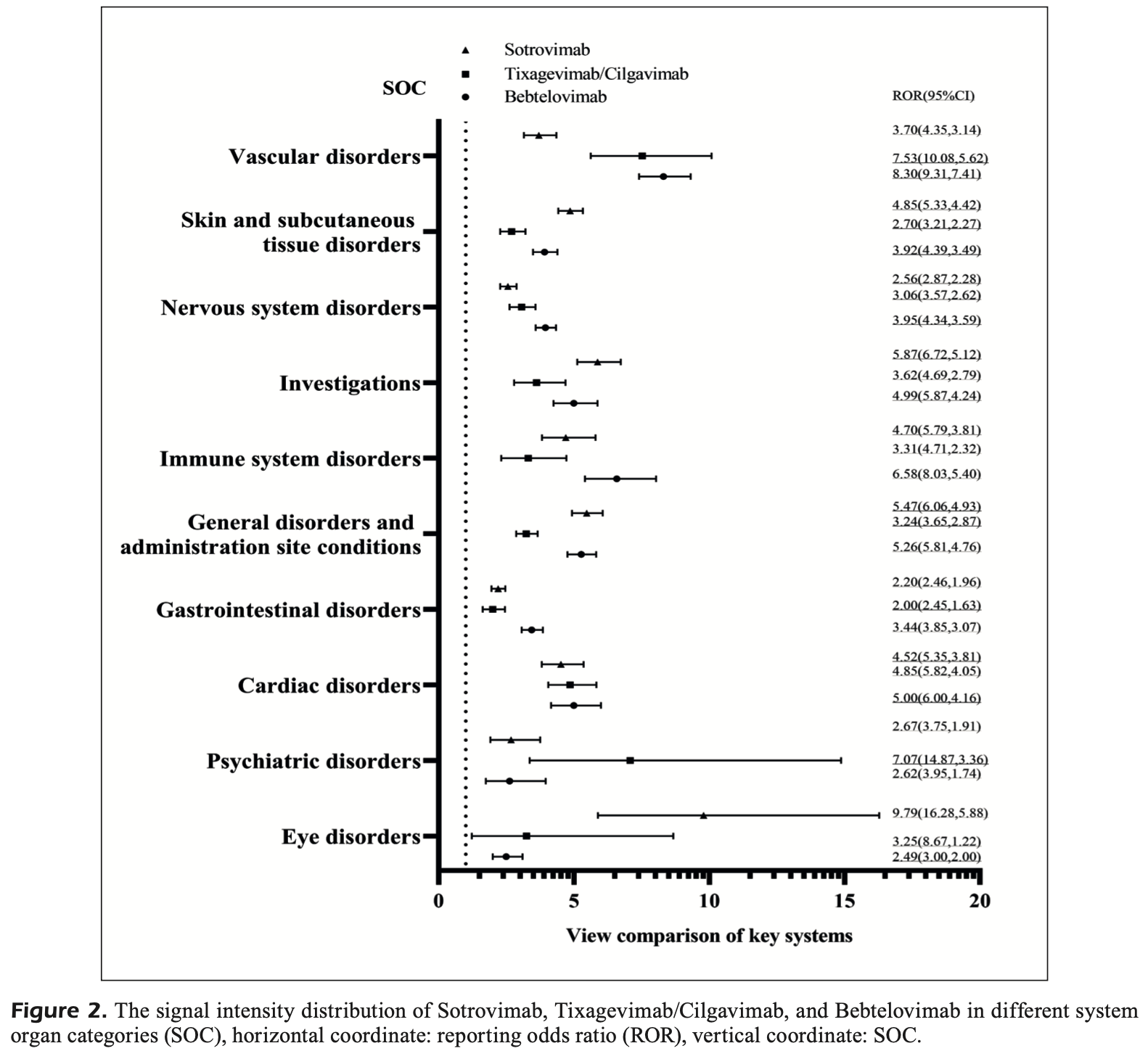
FDA adverse event reporting analysis for bebtelovimab, tixagevimab/cilgavimab, and sotrovimab, showing distinct safety profiles with concerning adverse events for each drug. Bebtelovimab showed the highest associations with gastrointestinal disorders (nausea) and nervous system disorders (dizziness), including severe eye complications like acute macular neuroretinopathy. Tixagevimab/cilgavimab was associated with multiple cardiovascular events including thrombosis, heart failure, and arrhythmias. Sotrovimab showed the strongest correlations with skin reactions, infusion-related hypersensitivity reactions, and anaphylactic shock. Authors hypothesize that sotrovimab's broader cross-coronavirus neutralization capacity may explain its stronger immunoreactivity compared to the other monoclonal antibodies.
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for omicron BA.2.75.2, BA.4.6, BQ.1.11, BA.5, BA.2.75, XBB2,3, XBB.1.53, ХВВ.1.9.13, XBB.1.9.3, XBB.1.5.24, XBB.1.16, XBB.2.9, BQ.1.1.45, CL.1, and CH.1.14.
Study covers sotrovimab, bebtelovimab, and tixagevimab/cilgavimab.
1.
Planas et al., Resistance of Omicron subvariants BA.2.75.2, BA.4.6 and BQ.1.1 to neutralizing antibodies, bioRxiv, doi:10.1101/2022.11.17.516888.
2.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
Wang et al., 30 Apr 2024, peer-reviewed, 4 authors.
Contact: linan270013@163.com.
OBJECTIVE: Bebtelovimab (BEB), Tixagevimab/Cilgavimab (TIX/CIL), and Sotrovimab (SOT) are important agents against the severe acute respiratory syndrome coronavirus 2-Omicron strain. However, due to their short duration of application, little is known about their safety profiles. This research aimed to explore the safety profile of these monoclonal antibodies (mAbs) via real-world evidence databases and data mining tools. MATERIALS AND METHODS: Safety reports were retrieved from the database of the U.S. Food and Drug Administration (FDA) Adverse Event Reporting System from April 2022 to March 2023. To detect the safety signal, the disproportionality analysis was performed using the reporting odds ratio method. RESULTS: SOT had the greatest proportion of "skin and subcutaneous tissue disorders" and "disorders of investigations"; BEB showed significant associations with "gastrointestinal disorders" and "nervous system disorders"; TIX/CIL had the weakest correlation with "skin and subcutaneous tissue disorders" and "general disorders and administration site conditions". Furthermore, there were still other signals related to nervous system disorders, gastrointestinal disorders only caused by BEB. TIX/ CIL has been reported solely to be associated with multiple types of cardiovascular disorders. As for SOT alone, signals were strongly related to infusion reactions and hypersensitivity. CONCLUSIONS: In summary, SOT may be unsuitable for allergic patients and may lead to abnormal test results. BEB showed the highest correlations with gastrointestinal and neuropsychiatric events. In addition, its infusion reactions should also be noted. TIX/CIL can lead to a variety of cardiovascular events.
Conflict of Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Informed Consent All the data in this study were sourced from The U.S. Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS), an open-access database. Therefore, informed consent is not applicable.
Ethics Approval All the data in this study was sourced from The U.S. Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS), which is an open-access database. Therefore, ethics approval is not applicable.
Authors' Contributions
References
Birabaharan, Hill, Begur, Kaelber, Martin et al., Cardiovascular Outcomes After Tixagevimab and Cilgavimab use for Pre-Exposure Prophylaxis Against Coronavirus Disease 2019: A Population-Based Propensi-ty-matched Cohort Study, Clin Infect Dis
Conti, Pregliasco, Calvisi, Caraffa, Gallenga et al., Monoclonal antibody therapy in COVID-19, J Biol Regul Homeost Agents
Glinianowicz, Ciura, Burnatowska, Olszanecka-Glinianowicz, Psychological effects of the COVID-19 pandemic -what do we know about them?, Eur Rev Med Pharmacol Sci
Guan, Ji, Zheng, Yang, Qin et al., Development of a drug risk analysis and assessment system and its application in signal excavation and analysis of 263 cases of fluoroquinolone-induced adverse reactions, Front Pharmacol
Gupta, Gonzalez-Rojas, Juarez, Casal, Moya et al., Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab, N Engl J Med
Iketani, Liu, Guo, Liu, Chan et al., Antibody evasion properties of SARS-CoV-2 Omicron sublineages, Nature
Jahanshahlu, Rezaei, Monoclonal antibody as a potential anti-COVID-19, Biomed Pharmacother
Montastruc, Lafaurie, Flumian, De Canecaude, Increased reporting of venous and arterial thromboembolic events reported with tixagevimab-cilgavimab for coronavirus disease 2019, Clin Microbiol Infect
Parums, Editorial: A Rapid Global Increase in COVID-19 is Due to the Emergence of the EG.5 (Eris) Subvariant of Omicron SARS-CoV-2, Med Sci Monit
Peng, Xiao, Ottaviani, Stebbing, Wang, A real-world disproportionality analysis of FDA Adverse Event Reporting System (FAERS) events for baricitinib, Expert Opin Drug Saf
Peters, Strayer, Wald, Serious Infusion Reactions in Two Adolescents Receiving Bebtelovimab, Pediatr Infect Dis J
Picard, Galvão, Current Knowledge and Management of Hypersensitivity Reactions to Monoclonal Antibodies, J Allergy Clin Immunol Pract
Rothman, Lanes, Sacks, The reporting odds ratio and its advantages over the proportional reporting ratio, Pharmacoepidemiol Drug Saf
Sakaeda, Kadoyama, Okuno, Statin-associated muscular and renal adverse events: data mining of the public version of the FDA adverse event reporting system, PLoS One
Shu, He, Liu, Wu, Zhang, A Real-World Disproportionality Analysis of Olaparib: Data Mining of the Public Version of FDA Adverse Event Reporting System, Clin Epidemiol
Trillenberg, Sprenger, Machner, Sensitivity and specificity in signal detection with the reporting odds ratio and the information component, Pharmacoepidemiol Drug Saf
Verden, Dimbil, Kyle, Overstreet, Hoffman, Analysis of Spontaneous Postmarket Case Reports Submitted to the FDA Regarding Thromboembolic Adverse Events and JAK Inhibitors, Drug Saf
Westendorf, Žentelis, Wang, Foster, Vaillancourt et al., LY-CoV1404 (bebtelovimab) potently neutralizes SARS-CoV-2 variants, Cell Rep
Yoshida, Shiraishi, Tanaka, Sotrovimab use in Japanese inpatients with COVID-19: post-infusion adverse events, BMC Infect Dis
Zost, Gilchuk, Case, Binshtein, Chen et al., Potently neutralizing and protective human antibodies against SARS-CoV-2, Nature
Zost, Gilchuk, Chen, Case, Reidy et al., Rapid isolation and profiling of a diverse panel of human monoclonal antibodies targeting the SARS-CoV-2 spike protein, Nat Med
DOI record:
{
"DOI": "10.26355/eurrev_202404_35925",
"ISSN": "1128-3602, 2284-0729",
"URL": "https://doi.org/10.26355/eurrev_202404_35925",
"abstract": "OBJECTIVE: Bebtelovimab (BEB), Tixagevimab/Cilgavimab (TIX/CIL), and Sotrovimab (SOT) are important agents against the severe acute respiratory syndrome coronavirus 2-Omicron strain. However, due to their short duration of application, little is known about their safety profiles. This research aimed to explore the safety profile of these monoclonal antibodies (mAbs) via real-world evidence databases and data mining tools. MATERIALS AND METHODS: Safety reports were retrieved from the database of the U.S. Food and Drug Administration (FDA) Adverse Event Reporting System from April 2022 to March 2023. To detect the safety signal, the disproportionality analysis was performed using the reporting odds ratio method. RESULTS: SOT had the greatest proportion of “skin and subcutaneous tissue disorders” and “disorders of investigations”; BEB showed significant associations with “gastrointestinal disorders” and “nervous system disorders”; TIX/CIL had the weakest correlation with “skin and subcutaneous tissue disorders” and “general disorders and administration site conditions”. Furthermore, there were still other signals related to nervous system disorders, gastrointestinal disorders only caused by BEB. TIX/CIL has been reported solely to be associated with multiple types of cardiovascular disorders. As for SOT alone, signals were strongly related to infusion reactions and hypersensitivity. CONCLUSIONS: In summary, SOT may be unsuitable for allergic patients and may lead to abnormal test results. BEB showed the highest correlations with gastrointestinal and neuropsychiatric events. In addition, its infusion reactions should also be noted. TIX/CIL can lead to a variety of cardiovascular events.",
"author": [
{
"affiliation": [
{
"name": "Department of Pharmacy, Mianyang Central Hospital, School of Medicine, University of Electronic Science and Technology of China, Mianyang, Sichuan, China. linan270013@163.com"
}
],
"family": "Wang",
"given": "Y."
},
{
"family": "Xu",
"given": "X.-W."
},
{
"family": "Zhou",
"given": "S."
},
{
"family": "Li",
"given": "J.-N."
}
],
"container-title": "European Review for Medical and Pharmacological Sciences",
"issue": "7",
"issued": {
"date-parts": [
[
2024,
4
]
]
},
"language": "eng",
"medium": "JB",
"page": "2943–2954",
"publisher": "Verduci Editore s.r.l.",
"publisher-place": "IT",
"title": "The safety signal detection and analysis of monoclonal antibodies against SARS-CoV-2 based on real-world evidence – the suitable selectivity for different populations",
"type": "article-journal",
"volume": "28"
}
wang52
