Early and Delayed Administration of Azvudine on Mortality of Adult Patients With COVID-19: A Retrospective Study
et al., American Journal of Respiratory and Critical Care Medicine, doi:10.1164/ajrccm.2025.211.Abstracts.A3027, May 2025
Azvudine for COVID-19
48th treatment shown to reduce risk in
January 2023, now with p = 0.000000017 from 39 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
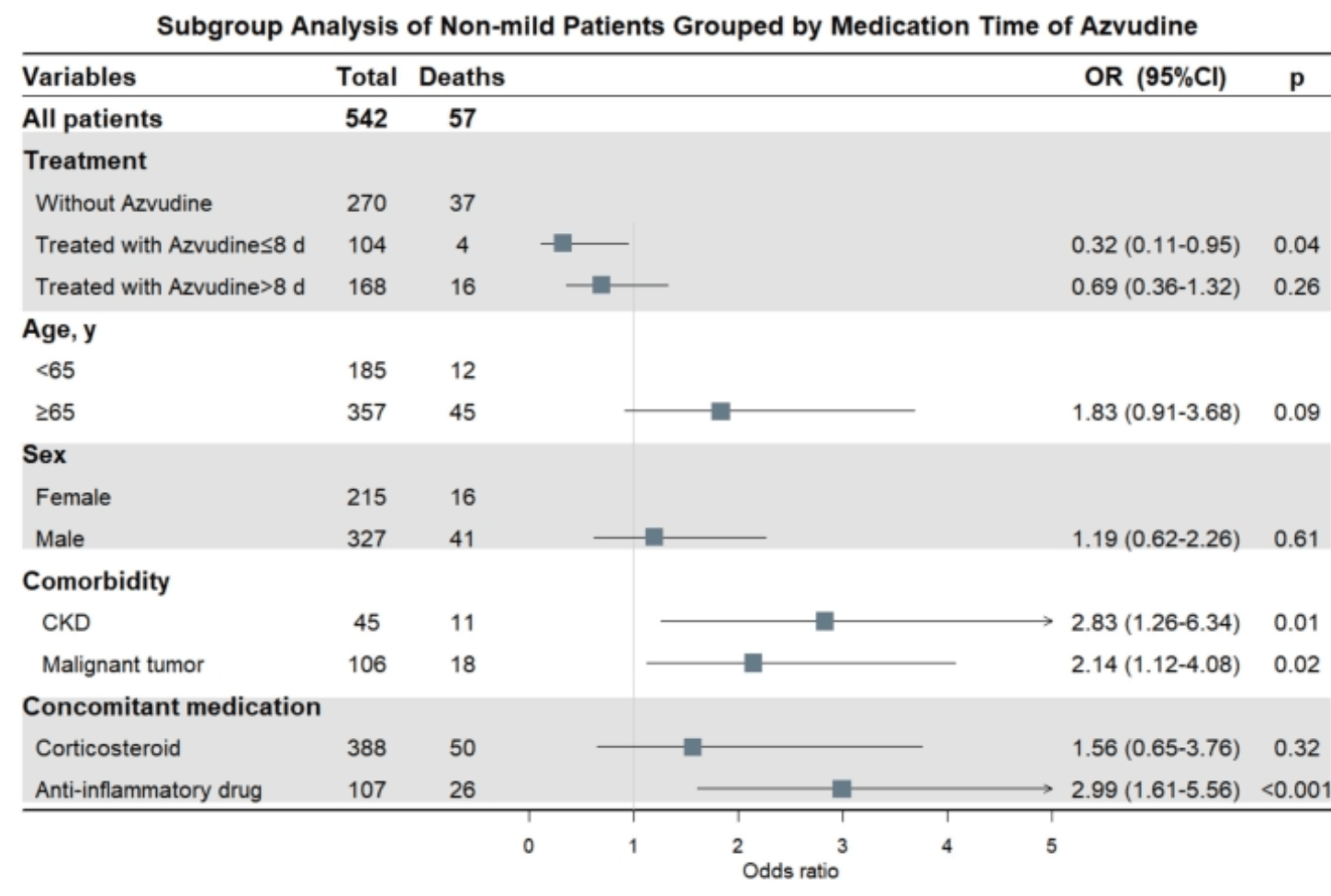
PSM retrospective 604 hospitalized COVID-19 patients showing lower mortality with azvudine. Detailed results are only provided for the subgroup of non-mild patients.
Wang et al., 7 May 2025, retrospective, China, preprint, 4 authors, study period 1 November, 2022 - 27 February, 2023.
Early and Delayed Administration of Azvudine on Mortality of Adult Patients With COVID-19: A Retrospective Study
RATIONALE: Azvudine is recommended for patients with coronavirus disease 19 ; however, its impact on mortality of patients and optimum therapeutic time window are unclear. The purpose of this study was to discuss the dosing window and compare the prognostic impact of azvudine use within and after the defined time window. METHODS: This was a single-centre, retrospective study conducted at the Peking Union Medical College Hospital (PUMCH) from 1 November 2022 to 27 February 2023 using 1:1 propensity score based on sex, age, comorbidities, and disease severity between patients receiving azvudine and inpatients without antiviral therapy. Clinical outcomes, including 28-day all-cause mortality and the incidence of 28-day disease progression, were assessed using univariate logistic regression analysis and adjusted for covariates through multivariate logistic regression analysis. RESULTS: A total of 421 COVID-19 patients were recorded receiving azvudine and 720 hospitalised patients with confirmed COVID-19 who had not used antiviral drugs at PUMCH. After propensity score matching, 302 patients treated with azvudine and 302 patients who were not treated with antiviral drugs were included. Compared with the latter, treatment with azvudine reduced the all-cause mortality rate by 51% to 28 days, and multivariate logistic regression analysis based on the time from symptom onset to medication showed that the use of azvudine will be significantly protective until 8 days of symptom onset for COVID-19 patients from death. Meanwhile, treatment with Azvudine within 8 days from symptom onset reduced the incidence of disease progression. CONCLUSIONS: The use of azvudine will reduce the risk of death in adult COVID-19 patients compared to without antiviral therapy and the benefit seems more significant within 8 days of symptoms onset.
