Treatment with sotrovimab for SARS-CoV-2 infection in a cohort of high-risk kidney transplant recipients
et al., Clinical Kidney Journal, doi:10.1093/ckj/sfac177, Jul 2022
Sotrovimab for COVID-19
45th treatment shown to reduce risk in
August 2022, now with p = 0.00048 from 29 studies, recognized in 42 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 82 kidney transplant recipients treated with sotrovimab, showing lower risk of serious COVID-19 outcomes with early treatment.
Efficacy is variant dependent. In Vitro studies predict lower efficacy for BA.11-3, BA.4, BA.54, XBB.1.9.3, XBB.1.5.24, XBB.2.9, CH.1.15, and no efficacy for BA.26, XBB, XBB.1.5, ХВВ.1.9.17, XBB.1.16, BQ.1.1.45, and CL.15. US EUA has been revoked.
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
4.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
5.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
Villanego et al., 28 Jul 2022, retrospective, Spain, peer-reviewed, median age 63.0, 25 authors, study period 1 December, 2021 - 28 February, 2022.
Contact: mauxiliadora.mazuecos.sspa@juntadeandalucia.es.
Treatment with sotrovimab for SARS-CoV-2 infection in a cohort of high-risk kidney transplant recipients
Clinical Kidney Journal, doi:10.1093/ckj/sfac177
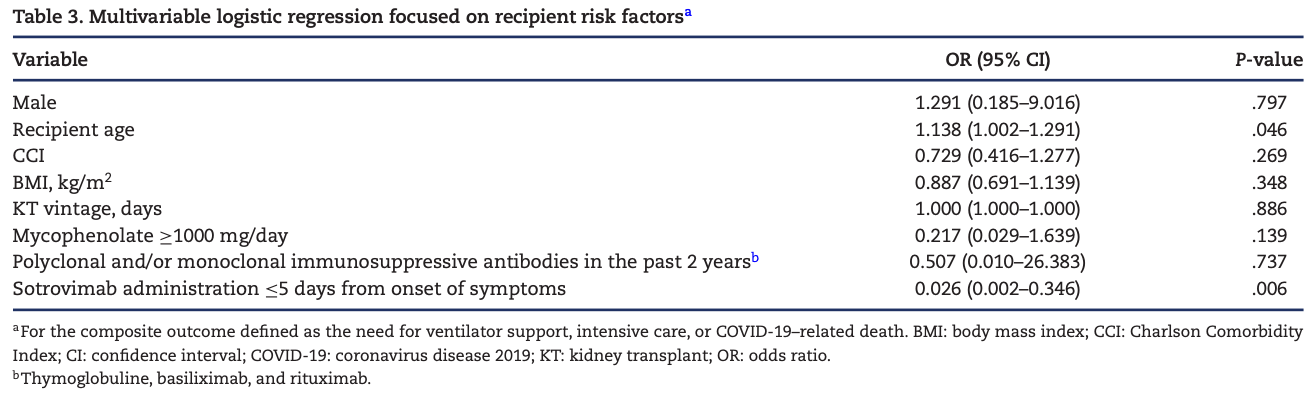
Background. Sotrovimab is a neutralizing monoclonal antibody (mAb) that seems to remain active against recent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants. The evidence on its use in kidney transplant (KT) recipients, however, is limited. Methods. We performed a multicenter, retrospective cohort study of 82 KT patients with SARS-CoV-2 infection {coronavirus disease 2019 [COVID-19]} treated with sotrovimab. Results. Median age was 63 years. Diabetes was present in 43.9% of patients, and obesity in 32.9% of patients; 48.8% of patients had an estimated glomerular filtration rate under 30 mL/minute/1.73 m 2 . Additional anti-COVID-19 therapies were administered to 56 patients, especially intravenous steroids (65.9%). Sotrovimab was administered early (<5 days from the onset of the symptoms) in 46 patients (56%). Early-treated patients showed less likely progression to severe COVID-19 than those treated later, represented as a lower need for ventilator support (2.2% vs 36.1%; P < .001) or intensive care admission (2.2% vs 25%; P = .002) and COVID-19-related mortality (2.2% vs 16.7%; P = .020). In the multivariable analysis, controlling for baseline risk factors to severe COVID-19 in KT recipients, early use of sotrovimab remained as a protective factor for a composite outcome, including need for ventilator support, intensive care, and COVID-19-related mortality. No anaphylactic reactions, acute rejection episodes, impaired kidney function events, or non-kidney side effects related to sotrovimab were observed. Conclusions. Sotrovimab had an excellent safety profile, even in high-comorbidity patients and advanced chronic kidney disease stages. Earlier administration could prevent progression to severe disease, while clinical outcomes were poor in patients treated later. Larger controlled studies enrolling KT recipients are warranted to elucidate the true efficacy of monoclonal antibody therapies.
SUPPLEMENTARY DATA Supplementary data are available at ckj online.
AUTHORS' CONTRIBUTIONS F.V., A.M., M.C., and J.P. designed the study, analyzed the data, and drafted the article. All authors revised the article, made substantial contributions, and approved the final version of the article. F.V. and A.M. have contributed equally to this work. M.C. and J.P. share senior authorship to this work.
CONFLICT OF INTEREST STATEMENT None declared.
References
Alkindi, Chaaban, Hakim, Sotrovimab use for COVID-19 infection in pregnant kidney transplant recipient, Transplantation
Angarone, Kumar, Stosor, Organ transplant patients, COVID-19, and neutralizing monoclonal antibodies: the glass is half full, Transpl Infect Dis
Angelico, Blasi, Manzia, The management of immunosuppression in kidney transplant recipients with COVID-19 disease: an update and systematic review of the literature, Medicina (Kaunas)
Buxeda, Arias-Cabrales, Mj, Use and safety of remdesivir in kidney transplant recipients with COVID-19, Kidney Int Rep
Callaghan, Mumford, Curtis, NGSBT Organ and Tissue Donation and Transplantation Clinical Team. Realworld effectiveness of the Pfizer-BioNTech BNT162b2 and Oxford-AstraZeneca ChAdOx1-S vaccines against SARS-CoV-2 in solid organ and islet transplant recipients, Transplantation
Case, Mackin, Errico, Resilience of S309 and AZD7442 monoclonal antibody treatments against infection by SARS-CoV-2 omicron lineage strains, Nat Commun
Chapman, Wigmore, Simple vaccination is not enough for the transplant recipient, Transplantation
Chavarot, Melenotte, Amrouche, Early treatment with sotrovimab monoclonal antibody in kidney transplant recipients with omicron infection, Kidney Int
Chen, Nirula, Heller, BLAZE-1 Investigators. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19, N Eng J Med
Chen, Wei, high potential to becoming the next dominating variant, Omicron BA, doi:10.21203/rs.3.rs-1362445/v1
Dhand, Okumura, Wolfe, Sotrovimab for treatment of COVID-19 in solid organ transplant recipients, Transplantation
Fernandes, Devresse, Scohy, Monoclonal antibody therapy for SARS-CoV-2 infection in kidney transplant recipients: a case series from Belgium, Transplantation
Fernandes, Devresse, Scohy, Monoclonal antibody therapy in kidney transplant recipients with delta and omicron variants of SARS-CoV-2: a single-center case series, Kidney Med
Gueguen, Colosio, Bello, Early administration of anti-SARS-CoV-2 monoclonal antibodies prevents severe COVID-19 in kidney transplant patients, Kidney Int Rep
Gupta, Gonzalez-Rojas, Juarez, COMET-ICE Investigators. Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab, N Eng J Med
Kawaoka, Uraki, Kiso, Characterization and antiviral susceptibility of SARS-CoV-2 omicron/BA.2, Res Sq, doi:10.21203/rs.3.rs-1375091/v1
Khwaja, KDIGO clinical practice guideline for acute kidney injury, Nephron Clin Pract
Kreuzberger, Hirsch, Chai, SARS-CoV-2-neutralising monoclonal antibodies for treatment of COVID-19, Cochrane Database Syst Rev
Kutzler, Kuzaro, Serrano, Initial experience of bamlanivimab monotherapy use in solid organ transplant recipients, Transpl Infect Dis
López, Vázquez, Alonso-Titos, de Estudio GREAT (Grupo Español de Actualizaciones en Trasplante). Recommendations on management of the SARS-CoV-2 coronavirus pandemic (Covid-19) in kidney transplant patients, Nefrologia (Engl Ed)
Mazuecos, Villanego, Zarraga, Spanish Society of Nephrology COVID-19 Group. Breakthrough infections following mRNA SARS-CoV-2 vaccination in kidney transplant recipients, Transplantation
Pinchera, Buonomo, Scotto, team. Sotrovimab in solid organ transplant patients with early, mild/moderate SARS-CoV-2 infection: a single-center experience, Transplantation
Qin, Moore, Anjan, Risk of breakthrough SARS-CoV-2 infections in adult transplant recipients, Transplantation
Quiroga, Soler, Ortiz, SENCOVAC collaborative network. Loss of humoral response 3 months after SARS-CoV-2 vaccination in the CKD spectrum: the multicentric SENCOVAC study, Nephrol Dial Transplant
Salerno, Jennings, Lange, Early clinical experience with nirmatrelvir/ritonavir for the treatment of COVID-19 in solid organ transplant recipients, Am J Transplant
Sarrell, Bloch, Chediak, Monoclonal antibody treatment for COVID-19 in solid organ transplant recipients, Transplant Infect Dis
Stumpf, Tonnus, Paliege, Cellular and humoral immune responses after 3 doses of BNT162b2 mRNA SARS-CoV-2 vaccine in kidney transplant, Transplantation
Takashita, Kinoshita, Yamayoshi, Efficacy of antiviral agents against the SARS-CoV-2 omicron subvariant BA.2, N Eng J Med
Villanego, Mazuecos, Pérez-Flores, Spanish Society of Nephrology COVID-19 Group. Predictors of severe COVID-19 in kidney transplant recipients in the different epidemic waves: analysis of the Spanish Registry, Am J Transplant
Yetmar, Beam, Horo, Monoclonal antibody therapy for COVID-19 in solid organ transplant recipients, Open Forum Infect Dis
Yetmar, Bhaimia, Bierle, Breakthrough COVID-19 after SARS-CoV-2 vaccination in solid organ transplant recipients: an analysis of symptomatic cases and monoclonal antibody therapy, Transpl Infect Dis
Zheng, Green, Tazare, Comparative effectiveness of sotrovimab and molnupiravir for prevention of severe COVID-19 outcomes in non-hospitalised patients: an observational cohort study using the OpenSAFELY platform, medRxiv, doi:10.1101/2022.05.22.22275417
DOI record:
{
"DOI": "10.1093/ckj/sfac177",
"ISSN": [
"2048-8505",
"2048-8513"
],
"URL": "http://dx.doi.org/10.1093/ckj/sfac177",
"abstract": "<jats:title>ABSTRACT</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Sotrovimab is a neutralizing monoclonal antibody (mAb) that seems to remain active against recent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants. The evidence on its use in kidney transplant (KT) recipients, however, is limited.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>We performed a multicenter, retrospective cohort study of 82 KT patients with SARS-CoV-2 infection {coronavirus disease 2019 [COVID-19]} treated with sotrovimab.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>Median age was 63 years. Diabetes was present in 43.9% of patients, and obesity in 32.9% of patients; 48.8% of patients had an estimated glomerular filtration rate under 30 mL/minute/1.73 m2. Additional anti–COVID-19 therapies were administered to 56 patients, especially intravenous steroids (65.9%). Sotrovimab was administered early (&lt;5 days from the onset of the symptoms) in 46 patients (56%). Early-treated patients showed less likely progression to severe COVID-19 than those treated later, represented as a lower need for ventilator support (2.2% vs 36.1%; P &lt; .001) or intensive care admission (2.2% vs 25%; P = .002) and COVID-19–related mortality (2.2% vs 16.7%; P = .020). In the multivariable analysis, controlling for baseline risk factors to severe COVID-19 in KT recipients, early use of sotrovimab remained as a protective factor for a composite outcome, including need for ventilator support, intensive care, and COVID-19–related mortality. No anaphylactic reactions, acute rejection episodes, impaired kidney function events, or non-kidney side effects related to sotrovimab were observed.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>Sotrovimab had an excellent safety profile, even in high-comorbidity patients and advanced chronic kidney disease stages. Earlier administration could prevent progression to severe disease, while clinical outcomes were poor in patients treated later. Larger controlled studies enrolling KT recipients are warranted to elucidate the true efficacy of monoclonal antibody therapies.</jats:p>\n </jats:sec>",
"author": [
{
"ORCID": "http://orcid.org/0000-0002-7828-9479",
"affiliation": [
{
"name": "Department of Nephrology, Hospital Universitario Puerta del Mar , Cádiz , Spain"
}
],
"authenticated-orcid": false,
"family": "Villanego",
"given": "Florentino",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-5860-2309",
"affiliation": [
{
"name": "Department of Nephrology, Hospital Universitario Puerta del Mar , Cádiz , Spain"
}
],
"authenticated-orcid": false,
"family": "Mazuecos",
"given": "Auxiliadora",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Nephrology, Hospital Clínico San Carlos , Madrid , Spain"
}
],
"family": "Cubillo",
"given": "Beatriz",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Nephrology, Hospital Universitario Juan Ramón Jiménez , Huelva , Spain"
}
],
"family": "Merino",
"given": "M José",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Nephrology, Hospital Universitario Torrecárdenas , Almería , Spain"
}
],
"family": "Poveda",
"given": "Inmaculada",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Nephrology, Hospital Clínico Universitario Virgen de la Arrixaca , Murcia , Spain"
}
],
"family": "Saura",
"given": "Isabel M",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Nephrology, Hospital Universitario Puerta del Mar , Cádiz , Spain"
}
],
"family": "Segurado",
"given": "Óscar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Nephrology, Hospital General Universitario de Elche , Elche , Spain"
}
],
"family": "Cruzado",
"given": "Leónidas",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Nephrology, Hospital Universitario de Jerez de la Frontera, Jerez de la Frontera , Spain"
}
],
"family": "Eady",
"given": "Myriam",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Nephrology, Hospital Universitario de Cruces , Bilbao , Spain"
}
],
"family": "Zárraga",
"given": "Sofía",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Nephrology, Hospital Universitario Miguel Servet , Zaragoza, Spain"
}
],
"family": "Aladrén",
"given": "M José",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Nephrology, Hospital Universitario Son Espases , Palma de Mallorca, Spain"
}
],
"family": "Cabello",
"given": "Sheila",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Nephrology, Hospital Regional Universitario de Málaga, Universidad de Málaga, Instituto de Investigación Biomédica de Málaga, REDinREN (RD16/0009/0006) , Málaga , Spain"
}
],
"family": "López",
"given": "Verónica",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Nephrology, Hospital Universitario Doce de Octubre, Institute i+12 for Medical Research , Madrid , Spain"
}
],
"family": "González",
"given": "Esther",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Nephrology, Complejo Hospitalario Universitario de Albacete , Albacete , Spain"
}
],
"family": "Lorenzo",
"given": "Inmaculada",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Nephrology, Hospital Universitario y Politécnico La Fe , Valencia , Spain"
}
],
"family": "Espí-Reig",
"given": "Jordi",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2801-6752",
"affiliation": [
{
"name": "Department of Nephrology, Complexo Hospitalario Universitario de A Coruña , A Coruña , Spain"
}
],
"authenticated-orcid": false,
"family": "Fernández",
"given": "Constantino",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Nephrology, Hospital Universitario Doctor Peset , Valencia , Spain"
}
],
"family": "Osma",
"given": "July",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Nephrology, Hospital Universitario Virgen de las Nieves , Granada , Spain"
}
],
"family": "Ruiz-Fuentes",
"given": "M Carmen",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5983-6863",
"affiliation": [
{
"name": "Department of Nephrology, Hospital Vall d´Hebron , Barcelona , Spain"
}
],
"authenticated-orcid": false,
"family": "Toapanta",
"given": "Néstor",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Nephrology, Hospital General Universitario de Alicante , Alicante , Spain"
}
],
"family": "Franco",
"given": "Antonio",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Nephrology, Hospital del Mar, Hospital del Mar Medical Research Institute, REDinREN (RD16/0009/0013) , Barcelona , Spain"
}
],
"family": "Burballa",
"given": "Carla C",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Nephrology, Hospital Universitario de Toledo , Toledo , Spain"
}
],
"family": "Muñoz",
"given": "Miguel A",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Nephrology, Hospital del Mar, Hospital del Mar Medical Research Institute, REDinREN (RD16/0009/0013) , Barcelona , Spain"
}
],
"family": "Crespo",
"given": "Marta",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Nephrology, Hospital Universitario Puerta del Mar , Cádiz , Spain"
},
{
"name": "Department of Nephrology, Hospital Clínico San Carlos , Madrid , Spain"
}
],
"family": "Pascual",
"given": "Julio",
"sequence": "additional"
}
],
"container-title": "Clinical Kidney Journal",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
7,
28
]
],
"date-time": "2022-07-28T18:52:47Z",
"timestamp": 1659034367000
},
"deposited": {
"date-parts": [
[
2022,
9,
22
]
],
"date-time": "2022-09-22T09:50:25Z",
"timestamp": 1663840225000
},
"funder": [
{
"DOI": "10.13039/100015883",
"doi-asserted-by": "publisher",
"name": "Spanish Society of Nephrology"
}
],
"indexed": {
"date-parts": [
[
2022,
9,
23
]
],
"date-time": "2022-09-23T05:45:55Z",
"timestamp": 1663911955439
},
"is-referenced-by-count": 0,
"issue": "10",
"issued": {
"date-parts": [
[
2022,
7,
28
]
]
},
"journal-issue": {
"issue": "10",
"published-online": {
"date-parts": [
[
2022,
7,
28
]
]
},
"published-print": {
"date-parts": [
[
2022,
9,
22
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
7,
28
]
],
"date-time": "2022-07-28T00:00:00Z",
"timestamp": 1658966400000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/ckj/advance-article-pdf/doi/10.1093/ckj/sfac177/45505732/sfac177.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/ckj/article-pdf/15/10/1847/45972170/sfac177.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/ckj/article-pdf/15/10/1847/45972170/sfac177.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"page": "1847-1855",
"prefix": "10.1093",
"published": {
"date-parts": [
[
2022,
7,
28
]
]
},
"published-online": {
"date-parts": [
[
2022,
7,
28
]
]
},
"published-other": {
"date-parts": [
[
2022,
10
]
]
},
"published-print": {
"date-parts": [
[
2022,
9,
22
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference": [
{
"DOI": "10.1097/TP.0000000000003907",
"article-title": "Risk of breakthrough SARS-CoV-2 infections in adult transplant recipients",
"author": "Qin",
"doi-asserted-by": "crossref",
"first-page": "e265",
"journal-title": "Transplantation",
"key": "2022092209482088600_bib1",
"volume": "105",
"year": "2021"
},
{
"DOI": "10.1097/TP.0000000000004119",
"article-title": "Breakthrough infections following mRNA SARS-CoV-2 vaccination in kidney transplant recipients",
"author": "Mazuecos",
"doi-asserted-by": "crossref",
"first-page": "1430",
"journal-title": "Transplantation",
"key": "2022092209482088600_bib2",
"volume": "106",
"year": "2022"
},
{
"DOI": "10.1097/TP.0000000000003903",
"article-title": "Cellular and humoral immune responses after 3 doses of BNT162b2 mRNA SARS-CoV-2 vaccine in kidney transplant",
"author": "Stumpf",
"doi-asserted-by": "crossref",
"first-page": "e267",
"journal-title": "Transplantation",
"key": "2022092209482088600_bib3",
"volume": "105",
"year": "2021"
},
{
"DOI": "10.1093/ndt/gfac007",
"article-title": "Loss of humoral response 3 months after SARS-CoV-2 vaccination in the CKD spectrum: the multicentric SENCOVAC study",
"author": "Quiroga",
"doi-asserted-by": "crossref",
"first-page": "994",
"journal-title": "Nephrol Dial Transplant",
"key": "2022092209482088600_bib4",
"volume": "37",
"year": "2022"
},
{
"DOI": "10.1097/TP.0000000000004059",
"article-title": "Real-world effectiveness of the Pfizer-BioNTech BNT162b2 and Oxford-AstraZeneca ChAdOx1-S vaccines against SARS-CoV-2 in solid organ and islet transplant recipients",
"author": "Callaghan",
"doi-asserted-by": "crossref",
"first-page": "436",
"journal-title": "Transplantation",
"key": "2022092209482088600_bib5",
"volume": "106",
"year": "2022"
},
{
"DOI": "10.1097/TP.0000000000004064",
"article-title": "Simple vaccination is not enough for the transplant recipient",
"author": "Chapman",
"doi-asserted-by": "crossref",
"first-page": "447",
"journal-title": "Transplantation",
"key": "2022092209482088600_bib6",
"volume": "106",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2029849",
"article-title": "SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "1382",
"journal-title": "N Eng J Med",
"key": "2022092209482088600_bib7",
"volume": "384",
"year": "2021"
},
{
"article-title": "SARS-CoV-2-neutralising monoclonal antibodies for treatment of COVID-19",
"author": "Kreuzberger",
"first-page": "CD013825",
"journal-title": "Cochrane Database Syst Rev",
"key": "2022092209482088600_bib8",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2107934",
"article-title": "Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab",
"author": "Gupta",
"doi-asserted-by": "crossref",
"first-page": "1941",
"journal-title": "N Eng J Med",
"key": "2022092209482088600_bib9",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1111/tid.13724",
"article-title": "Organ transplant patients, COVID-19, and neutralizing monoclonal antibodies: the glass is half full",
"author": "Angarone",
"doi-asserted-by": "crossref",
"first-page": "e13724",
"journal-title": "Transpl Infect Dis",
"key": "2022092209482088600_bib10",
"volume": "23",
"year": "2021"
},
{
"DOI": "10.1093/ofid/ofab255",
"article-title": "Monoclonal antibody therapy for COVID-19 in solid organ transplant recipients",
"author": "Yetmar",
"doi-asserted-by": "crossref",
"first-page": "ofab255",
"journal-title": "Open Forum Infect Dis",
"key": "2022092209482088600_bib11",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1111/tid.13662",
"article-title": "Initial experience of bamlanivimab monotherapy use in solid organ transplant recipients",
"author": "Kutzler",
"doi-asserted-by": "crossref",
"first-page": "e13662",
"journal-title": "Transpl Infect Dis",
"key": "2022092209482088600_bib12",
"volume": "23",
"year": "2021"
},
{
"DOI": "10.1097/TP.0000000000003974",
"article-title": "Monoclonal antibody therapy for SARS-CoV-2 infection in kidney transplant recipients: a case series from Belgium",
"author": "Fernandes",
"doi-asserted-by": "crossref",
"first-page": "e107",
"journal-title": "Transplantation",
"key": "2022092209482088600_bib13",
"volume": "106",
"year": "2022"
},
{
"DOI": "10.1111/tid.13759",
"article-title": "Monoclonal antibody treatment for COVID-19 in solid organ transplant recipients",
"author": "Sarrell",
"doi-asserted-by": "crossref",
"first-page": "e13759",
"journal-title": "Transplant Infect Dis",
"key": "2022092209482088600_bib14",
"volume": "24",
"year": "2022"
},
{
"DOI": "10.1016/j.ekir.2022.03.020",
"article-title": "Early administration of anti–SARS-CoV-2 monoclonal antibodies prevents severe COVID-19 in kidney transplant patients",
"author": "Gueguen",
"doi-asserted-by": "crossref",
"first-page": "1241",
"journal-title": "Kidney Int Rep",
"key": "2022092209482088600_bib15",
"volume": "7",
"year": "2022"
},
{
"DOI": "10.1111/tid.13779",
"article-title": "Breakthrough COVID-19 after SARS-CoV-2 vaccination in solid organ transplant recipients: an analysis of symptomatic cases and monoclonal antibody therapy",
"author": "Yetmar",
"doi-asserted-by": "crossref",
"first-page": "e13779",
"journal-title": "Transpl Infect Dis",
"key": "2022092209482088600_bib16",
"volume": "24",
"year": "2022"
},
{
"DOI": "10.1097/TP.0000000000004083",
"article-title": "Sotrovimab use for COVID-19 infection in pregnant kidney transplant recipient",
"author": "AlKindi",
"doi-asserted-by": "crossref",
"first-page": "e277",
"journal-title": "Transplantation",
"key": "2022092209482088600_bib17",
"volume": "106",
"year": "2022"
},
{
"DOI": "10.1097/TP.0000000000004150",
"article-title": "Sotrovimab in solid organ transplant patients with early, mild/moderate SARS-CoV-2 infection: a single-center experience",
"author": "Pinchera",
"doi-asserted-by": "crossref",
"first-page": "e343",
"journal-title": "Transplantation",
"key": "2022092209482088600_bib18",
"year": "2022"
},
{
"DOI": "10.1097/TP.0000000000004136",
"article-title": "Sotrovimab for treatment of COVID-19 in solid organ transplant recipients",
"author": "Dhand",
"doi-asserted-by": "crossref",
"first-page": "e336",
"journal-title": "Transplantation",
"key": "2022092209482088600_bib19",
"year": "2022"
},
{
"DOI": "10.1016/j.kint.2022.04.003",
"article-title": "Early treatment with sotrovimab monoclonal antibody in kidney transplant recipients with omicron infection",
"author": "Chavarot",
"doi-asserted-by": "crossref",
"first-page": "1290",
"journal-title": "Kidney Int",
"key": "2022092209482088600_bib20",
"volume": "101",
"year": "2022"
},
{
"DOI": "10.1016/j.xkme.2022.100470",
"article-title": "Monoclonal antibody therapy in kidney transplant recipients with delta and omicron variants of SARS-CoV-2: a single-center case series",
"author": "Fernandes",
"doi-asserted-by": "crossref",
"first-page": "100470",
"journal-title": "Kidney Med",
"key": "2022092209482088600_bib21",
"volume": "4",
"year": "2022"
},
{
"author": "Spanish Agency of Medicines and Medical Devices. Meeting of the Committee for Medicinal Products for Human Use in December 2021 [in Spanish]. January 4, 2022.",
"key": "2022092209482088600_bib22"
},
{
"author": "Spanish Agency of Medicines and Medical Devices",
"key": "2022092209482088600_bib23"
},
{
"author": "European Medicines Agency",
"key": "2022092209482088600_bib24"
},
{
"author": "Government of Spain Ministry of Health",
"key": "2022092209482088600_bib25"
},
{
"author": "The Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC)",
"key": "2022092209482088600_bib26"
},
{
"DOI": "10.1159/000339789",
"article-title": "KDIGO clinical practice guideline for acute kidney injury",
"author": "Khwaja",
"doi-asserted-by": "crossref",
"first-page": "c179",
"journal-title": "Nephron Clin Pract",
"key": "2022092209482088600_bib27",
"volume": "120",
"year": "2012"
},
{
"DOI": "10.1016/j.nefroe.2020.03.017",
"article-title": "Recommendations on management of the SARS-CoV-2 coronavirus pandemic (Covid-19) in kidney transplant patients",
"author": "López",
"doi-asserted-by": "crossref",
"first-page": "265",
"journal-title": "Nefrologia (Engl Ed)",
"key": "2022092209482088600_bib28",
"volume": "40",
"year": "2020"
},
{
"DOI": "10.3390/medicina57050435",
"article-title": "The management of immunosuppression in kidney transplant recipients with COVID-19 disease: an update and systematic review of the literature",
"author": "Angelico",
"doi-asserted-by": "crossref",
"first-page": "435",
"journal-title": "Medicina (Kaunas)",
"key": "2022092209482088600_bib29",
"volume": "57",
"year": "2021"
},
{
"DOI": "10.1111/ajt.16579",
"article-title": "Predictors of severe COVID-19 in kidney transplant recipients in the different epidemic waves: analysis of the Spanish Registry",
"author": "Villanego",
"doi-asserted-by": "crossref",
"first-page": "2573",
"journal-title": "Am J Transplant",
"key": "2022092209482088600_bib30",
"volume": "21",
"year": "2021"
},
{
"article-title": "Efficacy and safety of two neutralising monoclonal antibody therapies, sotrovimab and BRII-196 plus BRII-198, for adults hospitalised with COVID-19 (TICO): a randomised controlled trial",
"author": "ACTIV-3/Therapeutics for Inpatients with COVID-19 (TICO) Study Group",
"first-page": "622",
"journal-title": "Lancet Infect Dis.",
"key": "2022092209482088600_bib31",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1016/j.ekir.2021.06.023",
"article-title": "Use and safety of remdesivir in kidney transplant recipients with COVID-19",
"author": "Buxeda",
"doi-asserted-by": "crossref",
"first-page": "2305",
"journal-title": "Kidney Int Rep",
"key": "2022092209482088600_bib32",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1111/ajt.17027",
"article-title": "Early clinical experience with nirmatrelvir/ritonavir for the treatment of COVID-19 in solid organ transplant recipients",
"author": "Salerno",
"doi-asserted-by": "crossref",
"first-page": "2083",
"journal-title": "Am J Transplant",
"key": "2022092209482088600_bib33",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1101/2022.05.22.22275417",
"article-title": "Comparative effectiveness of sotrovimab and molnupiravir for prevention of severe COVID-19 outcomes in non-hospitalised patients: an observational cohort study using the OpenSAFELY platform",
"author": "Zheng",
"doi-asserted-by": "crossref",
"key": "2022092209482088600_bib34"
},
{
"DOI": "10.1056/NEJMc2201933",
"article-title": "Efficacy of antiviral agents against the SARS-CoV-2 omicron subvariant BA.2",
"author": "Takashita",
"doi-asserted-by": "crossref",
"first-page": "1475",
"journal-title": "N Eng J Med",
"key": "2022092209482088600_bib35",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1038/s41467-022-31615-7",
"article-title": "Resilience of S309 and AZD7442 monoclonal antibody treatments against infection by SARS-CoV-2 omicron lineage strains",
"author": "Case",
"doi-asserted-by": "crossref",
"first-page": "3824",
"journal-title": "Nat Commun",
"key": "2022092209482088600_bib36",
"volume": "13",
"year": "2022"
},
{
"article-title": "Characterization and antiviral susceptibility of SARS-CoV-2 omicron/BA.2",
"author": "Kawaoka",
"journal-title": "Res Sq",
"key": "2022092209482088600_bib37"
},
{
"article-title": "Omicron BA.2 (B.1.1.529.2): high potential to becoming the next dominating variant",
"author": "Chen",
"journal-title": "Res Sq",
"key": "2022092209482088600_bib38"
},
{
"author": "US Food and Drug Administration",
"key": "2022092209482088600_bib39"
},
{
"author": "National Institutes of Health COVID-19 Treatment Guidelines",
"key": "2022092209482088600_bib40"
}
],
"reference-count": 40,
"references-count": 40,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/ckj/article/15/10/1847/6651282"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Transplantation",
"Nephrology"
],
"subtitle": [],
"title": "Treatment with sotrovimab for SARS-CoV-2 infection in a cohort of high-risk kidney transplant recipients",
"type": "journal-article",
"volume": "15"
}
