Convalescent plasma in the treatment of moderate to severe COVID-19 pneumonia: a randomized controlled trial (PROTECT-Patient Trial)
et al., Scientific Reports, doi:10.1038/s41598-022-06221-8, PROTECT-Patient, NCT04516811, Feb 2022
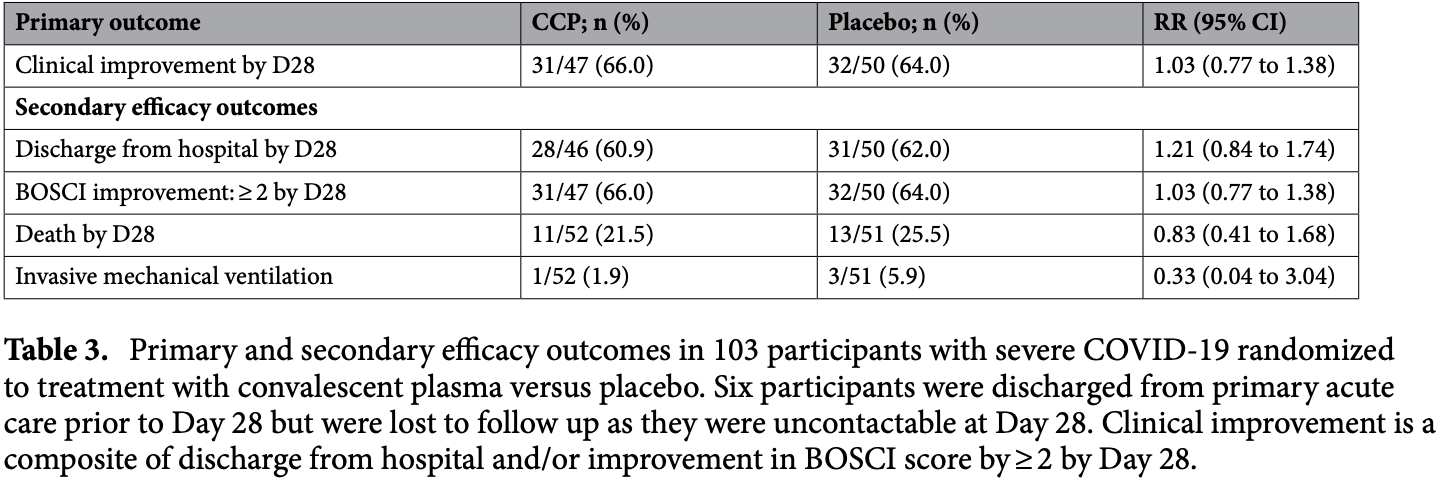
RCT 103 hospitalized patients in South Africa, showing no significant difference in outcomes with convalescent plasma.
|
risk of death, 17.0% lower, RR 0.83, p = 0.65, treatment 11 of 52 (21.2%), control 13 of 51 (25.5%), NNT 23, day 28.
|
|
risk of mechanical ventilation, 67.3% lower, RR 0.33, p = 0.36, treatment 1 of 52 (1.9%), control 3 of 51 (5.9%), NNT 25.
|
|
risk of no improvement, 5.4% lower, RR 0.95, p = 1.00, treatment 16 of 47 (34.0%), control 18 of 50 (36.0%), NNT 51, day 28.
|
|
risk of no hospital discharge, 3.0% higher, RR 1.03, p = 1.00, treatment 18 of 46 (39.1%), control 19 of 50 (38.0%), day 28.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
van den Berg et al., 15 Feb 2022, Randomized Controlled Trial, placebo-controlled, South Africa, peer-reviewed, 30 authors, study period 30 September, 2020 - 14 January, 2021, average treatment delay 9.0 days, trial NCT04516811 (history) (PROTECT-Patient).
Contact: karin.vandenberg@sanbs.org.za.
Convalescent plasma in the treatment of moderate to severe COVID-19 pneumonia: a randomized controlled trial (PROTECT-Patient Trial)
Scientific Reports, doi:10.1038/s41598-022-06221-8
There is a need for effective therapy for COVID-19 pneumonia. Convalescent plasma has antiviral activity and early observational studies suggested benefit in reducing COVID-19 severity. We investigated the safety and efficacy of convalescent plasma in hospitalized patients with COVID-19 in a population with a high HIV prevalence and where few therapeutic options were available. We performed a double-blinded, multicenter, randomized controlled trial in one private and three public sector hospitals in South Africa. Adult participants with COVID-19 pneumonia requiring non-invasive oxygen were randomized 1:1 to receive a single transfusion of 200 mL of either convalescent plasma
www.nature.com/scientificreports/ definitive conclusions, absence of a major safety signal is somewhat reassuring. In line with experience in other settings 11 , CCP use was safe in our overall cohort, supporting future evaluation for different indications in high HIV burden populations. The premature termination of the PROTECT trial illustrates the complexity of undertaking clinical research in a rapidly evolving global pandemic. Inconsistent case numbers, viral evolution, and rapidly changing evidence during the trial period contributed to this challenge. Despite this, our trial demonstrated the feasibility of deploying CCP in a resource-limited setting and contributed knowledge on the use of this therapeutic strategy for COVID-19 pneumonia. Our experience highlights the necessity of globally networked clinical trial sites and harmonised study protocols to more efficiently evaluate interventions among diverse populations during a pandemic.
Competing interests The authors declare no competing interests.
References
Agarwal, Convalescent plasma in the management of moderate covid-19 in adults in India: Open label phase II multicentre randomised controlled trial (PLACID Trial), BMJ
Amanat, A serological assay to detect SARS-CoV-2 seroconversion in humans, Nat. Med
Axfors, Association between convalescent plasma treatment and mortality in COVID-19: A collaborative systematic review and meta-analysis of randomized clinical trials, BMC Infect. Dis
Bajpai, Kumar, Maheshwari, Efficacy of convalescent plasma therapy compared to fresh frozen plasma in severely ill COVID-19 patients: A pilot randomized controlled trial, medRxiv, doi:10.1101/2020.10.25
Beigel, Remdesivir for the Treatment of Covid-19-Final Report, N. Engl. J. Med
Bloch, Deployment of convalescent plasma for the prevention and treatment of COVID-19, J. Clin. Investig
Bloch, Guidance for the procurement of COVID-19 convalescent plasma: Differences between high-and low-middleincome countries, Vox Sang
Casadevall, Dadachova, Pirofski, Passive antibody therapy for infectious diseases, Nat. Rev. Microbiol
Chalmers, Abo-Leyah, Loftus, Spears, Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial, Lancet
Dougan, Bamlanivimab plus Etesevimab in Mild or Moderate Covid-19, N. Engl. J. Med, doi:10.1056/NEJMoa2102685
Duan, Effectiveness of convalescent plasma therapy in severe COVID-19 patients, Proc. Natl. Acad. Sci. U. S. A
González, Regairaz, Salazar, Timing of convalescent plasma administration and 28-day mortality for COVID-19 pneumonia, medRxiv, doi:10.1101/2021.02.02
Guan, Clinical characteristics of coronavirus disease 2019 in China, N. Engl. J. Med
Harris, Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support, J. Biomed. Inform
Horby, Mafham, Peto, Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial
Horby, On behalf of the RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19, N. Engl. J. Med
Horny, of the RECOVERY Collaborative Group. Convalescent plasma in patients admitted to hospital with COVID-19: A randomised controlled, open-label, platform trial, Lancet
Huang, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet
Joyner, Convalescent plasma antibody levels and the risk of death from Covid-19, N. Engl. J. Med
Joyner, Safety update: COVID-19 Convalescent plasma in 20,000 hospitalized patients, Mayo Clin. Proc
Korley, Early convalescent plasma for high-risk outpatients with Covid-19, N. Engl. J. Med
Kyriazopoulou, Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: A double-blind, randomized controlled phase 3 trial, Nat. Med
Libster, Early high-titer plasma therapy to prevent severe Covid-19 in older adults, N. Engl. J. Med
Ling, Sim, Tan, Convalescent plasma for patients hospitalized with coronavirus disease 2019: A metaanalysis with trial sequential analysis of randomized controlled trials, Transfus Med Rev
Lundgren, On behalf of the Activ-Tico Ly-CoV555 Study Group. A neutralizing monoclonal antibody for hospitalized patients with Covid-19, N. Engl. J. Med
Mair-Jenkins, Saavedra-Campos, Baillie, The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: A systematic review and exploratory meta-analysis, J. Infect. Dis
Marconi, Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): A randomised, double-blind, parallel-group, placebo-controlled phase 3 trial, Lancet Respir. Med
Moyo-Gwete, Cross-reactive neutralizing antibody responses elicited by SARS-CoV-2 501Y.V2 (B.1.351, N. Engl. J. Med
O'donnell, A randomized double-blind controlled trial of convalescent plasma in adults with severe COVID-19, J. Clin. Investig
Recovery Collaborative Group, Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial, Lancet
Rojas, Convalescent plasma in Covid-19: Possible mechanisms of action, Autoimmun. Rev
Senefeld, Therapeutic use of convalescent plasma in COVID-19 patients with immunodeficiency, doi:10.1101/2020.11.08
Shen, Treatment of 5 critically ill patients with COVID-19 with convalescent plasma, JAMA
Simonovich, A randomized trial of convalescent plasma in Covid-19 severe pneumonia, N. Engl. J. Med
Tegally, Detection of a SARS-CoV-2 variant of concern in South Africa, Nature
Wibmer, SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma, Nat. Med
Zhou, Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study, Lancet
Zhou, Evidence of escape of SARS-CoV-2 variant B.1.351 from natural and vaccine-induced sera, Cell
DOI record:
{
"DOI": "10.1038/s41598-022-06221-8",
"ISSN": [
"2045-2322"
],
"URL": "http://dx.doi.org/10.1038/s41598-022-06221-8",
"abstract": "<jats:title>Abstract</jats:title><jats:p>There is a need for effective therapy for COVID-19 pneumonia. Convalescent plasma has antiviral activity and early observational studies suggested benefit in reducing COVID-19 severity. We investigated the safety and efficacy of convalescent plasma in hospitalized patients with COVID-19 in a population with a high HIV prevalence and where few therapeutic options were available. We performed a double-blinded, multicenter, randomized controlled trial in one private and three public sector hospitals in South Africa. Adult participants with COVID-19 pneumonia requiring non-invasive oxygen were randomized 1:1 to receive a single transfusion of 200 mL of either convalescent plasma or 0.9% saline solution. The primary outcome measure was hospital discharge and/or improvement of ≥ 2 points on the World Health Organisation Blueprint Ordinal Scale for Clinical Improvement by day 28 of enrolment. The trial was stopped early for futility by the Data and Safety Monitoring Board. 103 participants, including 21 HIV positive individuals, were randomized at the time of premature trial termination: 52 in the convalescent plasma and 51 in the placebo group. The primary outcome occurred in 31 participants in the convalescent plasma group and and 32 participants in the placebo group (relative risk 1.03 (95% CI 0.77 to 1.38). Two grade 1 transfusion-related adverse events occurred. Participants who improved clinically received convalescent plasma with a higher median anti-SARS-CoV-2 neutralizing antibody titre compared with those who did not (298 versus 205 AU/mL). Our study contributes additional evidence for recommendations against the use of convalescent plasma for COVID-19 pneumonia. Safety and feasibility in this population supports future investigation for other indications.</jats:p>",
"alternative-id": [
"6221"
],
"article-number": "2552",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "5 October 2021"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "24 January 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "15 February 2022"
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1,
"value": "The authors declare no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "van den Berg",
"given": "Karin",
"sequence": "first"
},
{
"affiliation": [],
"family": "Glatt",
"given": "Tanya Nadia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vermeulen",
"given": "Marion",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Little",
"given": "Francesca",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Swanevelder",
"given": "Ronel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Barrett",
"given": "Claire",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Court",
"given": "Richard",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bremer",
"given": "Marise",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nyoni",
"given": "Cynthia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Swarts",
"given": "Avril",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mmenu",
"given": "Cordelia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Crede",
"given": "Thomas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kritzinger",
"given": "Gerdien",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Naude",
"given": "Jonathan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Szymanski",
"given": "Patryk",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cowley",
"given": "James",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moyo-Gwete",
"given": "Thandeka",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moore",
"given": "Penny L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Black",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Singh",
"given": "Jaimendra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bhiman",
"given": "Jinal N.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Baijnath",
"given": "Prinita",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mody",
"given": "Priyesh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Malherbe",
"given": "Jacques",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Potgieter",
"given": "Samantha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "van Vuuren",
"given": "Cloete",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Maasdorp",
"given": "Shaun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wilkinson",
"given": "Robert J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Louw",
"given": "Vernon J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wasserman",
"given": "Sean",
"sequence": "additional"
}
],
"container-title": "Scientific Reports",
"container-title-short": "Sci Rep",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2022,
2,
15
]
],
"date-time": "2022-02-15T11:03:01Z",
"timestamp": 1644922981000
},
"deposited": {
"date-parts": [
[
2022,
11,
24
]
],
"date-time": "2022-11-24T23:07:16Z",
"timestamp": 1669331236000
},
"funder": [
{
"award": [
"K43TW011421"
],
"name": "National Institute of Health, South Africa"
},
{
"award": [
"1D43-TW010345"
],
"name": "NIH Fogarty International Center Training Grant"
},
{
"award": [
"98341"
],
"name": "South African Research Chairs Initiative of the Department of Science and Innovation and the National Research Foundation"
},
{
"DOI": "10.13039/501100000289",
"award": [
"FC0010218"
],
"doi-asserted-by": "publisher",
"name": "Cancer Research UK"
},
{
"DOI": "10.13039/501100000265",
"award": [
"FC0010218"
],
"doi-asserted-by": "publisher",
"name": "Medical Research Council"
}
],
"indexed": {
"date-parts": [
[
2022,
12,
21
]
],
"date-time": "2022-12-21T02:35:13Z",
"timestamp": 1671590113233
},
"is-referenced-by-count": 11,
"issue": "1",
"issued": {
"date-parts": [
[
2022,
2,
15
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2022,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
2,
15
]
],
"date-time": "2022-02-15T00:00:00Z",
"timestamp": 1644883200000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
2,
15
]
],
"date-time": "2022-02-15T00:00:00Z",
"timestamp": 1644883200000
}
}
],
"link": [
{
"URL": "https://www.nature.com/articles/s41598-022-06221-8.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-022-06221-8",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-022-06221-8.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1038",
"published": {
"date-parts": [
[
2022,
2,
15
]
]
},
"published-online": {
"date-parts": [
[
2022,
2,
15
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1056/NEJMoa2021436",
"author": "P Horby",
"doi-asserted-by": "publisher",
"first-page": "693",
"issue": "8",
"journal-title": "N. Engl. J. Med.",
"key": "6221_CR1",
"unstructured": "Horby, P. et al. On behalf of the RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N. Engl. J. Med. 384(8), 693–704 (2021).",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(21)00676-0",
"author": "J Chalmers",
"doi-asserted-by": "publisher",
"first-page": "1637",
"issue": "10285",
"journal-title": "Lancet (London, England)",
"key": "6221_CR2",
"unstructured": "Chalmers, J., Abo-Leyah, H., Loftus, H. & Spears, M. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet (London, England) 397(10285), 1637–1645 (2021).",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(21)00676-0",
"author": "RECOVERY Collaborative Group*",
"doi-asserted-by": "publisher",
"first-page": "1637",
"journal-title": "Lancet (London, England)",
"key": "6221_CR3",
"unstructured": "RECOVERY Collaborative Group*. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet (London, England) 397, 1637–1645 (2021).",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(21)00331-3",
"author": "VC Marconi",
"doi-asserted-by": "publisher",
"first-page": "1407",
"journal-title": "Lancet Respir. Med.",
"key": "6221_CR4",
"unstructured": "Marconi, V. C. et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): A randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir. Med. 9, 1407–1418 (2021).",
"volume": "9",
"year": "2021"
},
{
"key": "6221_CR5",
"unstructured": "Horby, P. W., Mafham, M., Peto, L., et al. On behalf of the RECOVERY Collaborative Group. Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. medRxiv 2021.06.15.21258542 (2021)."
},
{
"DOI": "10.1038/s41591-021-01499-z",
"author": "E Kyriazopoulou",
"doi-asserted-by": "publisher",
"first-page": "1752",
"issue": "10",
"journal-title": "Nat. Med.",
"key": "6221_CR6",
"unstructured": "Kyriazopoulou, E. et al. Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: A double-blind, randomized controlled phase 3 trial. Nat. Med. 27(10), 1752–1760 (2021) (Erratum in: Nat Med. 2021 Oct 8).",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2007764",
"author": "JH Beigel",
"doi-asserted-by": "publisher",
"first-page": "1813",
"issue": "19",
"journal-title": "N. Engl. J. Med.",
"key": "6221_CR7",
"unstructured": "Beigel, J. H. et al. Remdesivir for the Treatment of Covid-19—Final Report. N. Engl. J. Med. 383(19), 1813–1826 (2020).",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1016/j.autrev.2020.102554",
"author": "M Rojas",
"doi-asserted-by": "publisher",
"first-page": "102554",
"issue": "7",
"journal-title": "Autoimmun. Rev.",
"key": "6221_CR8",
"unstructured": "Rojas, M. et al. Convalescent plasma in Covid-19: Possible mechanisms of action. Autoimmun. Rev. 19(7), 102554 (2020).",
"volume": "19",
"year": "2020"
},
{
"DOI": "10.1038/nrmicro974",
"author": "A Casadevall",
"doi-asserted-by": "publisher",
"first-page": "695",
"issue": "9",
"journal-title": "Nat. Rev. Microbiol.",
"key": "6221_CR9",
"unstructured": "Casadevall, A., Dadachova, E. & Pirofski, L. A. Passive antibody therapy for infectious diseases. Nat. Rev. Microbiol. 2(9), 695–703 (2004).",
"volume": "2",
"year": "2004"
},
{
"DOI": "10.1093/infdis/jiu396",
"author": "J Mair-Jenkins",
"doi-asserted-by": "publisher",
"first-page": "80",
"issue": "1",
"journal-title": "J. Infect. Dis.",
"key": "6221_CR10",
"unstructured": "Mair-Jenkins, J., Saavedra-Campos, M. & Baillie, J. K. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: A systematic review and exploratory meta-analysis. J. Infect. Dis. 211(1), 80–90 (2015).",
"volume": "211",
"year": "2015"
},
{
"DOI": "10.1016/j.mayocp.2020.06.028",
"author": "MJ Joyner",
"doi-asserted-by": "publisher",
"first-page": "1888",
"issue": "9",
"journal-title": "Mayo Clin. Proc.",
"key": "6221_CR11",
"unstructured": "Joyner, M. J. et al. Safety update: COVID-19 Convalescent plasma in 20,000 hospitalized patients. Mayo Clin. Proc. 95(9), 1888–1897 (2020).",
"volume": "95",
"year": "2020"
},
{
"DOI": "10.1111/vox.12970",
"author": "EM Bloch",
"doi-asserted-by": "publisher",
"first-page": "18",
"issue": "1",
"journal-title": "Vox Sang",
"key": "6221_CR12",
"unstructured": "Bloch, E. M. et al. Guidance for the procurement of COVID-19 convalescent plasma: Differences between high- and low-middle-income countries. Vox Sang 116(1), 18–35 (2021).",
"volume": "116",
"year": "2021"
},
{
"DOI": "10.1172/JCI138745",
"author": "EM Bloch",
"doi-asserted-by": "publisher",
"first-page": "2757",
"issue": "6",
"journal-title": "J. Clin. Investig.",
"key": "6221_CR13",
"unstructured": "Bloch, E. M. et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J. Clin. Investig. 130(6), 2757–2765 (2020).",
"volume": "130",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.4783",
"author": "C Shen",
"doi-asserted-by": "publisher",
"first-page": "1582",
"issue": "16",
"journal-title": "JAMA",
"key": "6221_CR14",
"unstructured": "Shen, C. et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA 323(16), 1582–1589 (2020).",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1073/pnas.2004168117",
"author": "K Duan",
"doi-asserted-by": "publisher",
"first-page": "9490",
"issue": "17",
"journal-title": "Proc. Natl. Acad. Sci. U. S. A.",
"key": "6221_CR15",
"unstructured": "Duan, K. et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. U. S. A. 117(17), 9490–9496 (2020).",
"volume": "117",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2031893",
"author": "MJ Joyner",
"doi-asserted-by": "publisher",
"first-page": "1015",
"issue": "11",
"journal-title": "N. Engl. J. Med.",
"key": "6221_CR16",
"unstructured": "Joyner, M. J. et al. Convalescent plasma antibody levels and the risk of death from Covid-19. N. Engl. J. Med. 384(11), 1015–1027 (2021).",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1101/2020.10.25",
"doi-asserted-by": "publisher",
"key": "6221_CR17",
"unstructured": "Bajpai, M., Kumar, S., Maheshwari, A., et al. Efficacy of convalescent plasma therapy compared to fresh frozen plasma in severely ill COVID-19 patients: A pilot randomized controlled trial. medRxiv 2020: 20219337 [Preprint]. August 25, 2021 (accessed 12 Aug 2021). https://doi.org/10.1101/2020.10.25"
},
{
"DOI": "10.1172/JCI150646",
"author": "MR O’Donnell",
"doi-asserted-by": "publisher",
"issue": "13",
"journal-title": "J. Clin. Investig.",
"key": "6221_CR18",
"unstructured": "O’Donnell, M. R. et al. A randomized double-blind controlled trial of convalescent plasma in adults with severe COVID-19. J. Clin. Investig. 131(13), e150646 (2021).",
"volume": "131",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(21)00897-7",
"author": "P Horny",
"doi-asserted-by": "publisher",
"first-page": "2049",
"issue": "10289",
"journal-title": "Lancet",
"key": "6221_CR19",
"unstructured": "Horny, P. et al. On behalf of the RECOVERY Collaborative Group. Convalescent plasma in patients admitted to hospital with COVID-19: A randomised controlled, open-label, platform trial. Lancet 397(10289), 2049–2059 (2021).",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1016/j.jbi.2008.08.010",
"author": "PA Harris",
"doi-asserted-by": "publisher",
"first-page": "377",
"issue": "2",
"journal-title": "J. Biomed. Inform.",
"key": "6221_CR20",
"unstructured": "Harris, P. A. et al. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 42(2), 377–381 (2009).",
"volume": "42",
"year": "2009"
},
{
"DOI": "10.1038/s41591-020-0913-5",
"author": "F Amanat",
"doi-asserted-by": "publisher",
"first-page": "1033",
"issue": "7",
"journal-title": "Nat. Med.",
"key": "6221_CR21",
"unstructured": "Amanat, F. et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat. Med. 26(7), 1033–1036 (2020).",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1056/NEJMc2104192",
"author": "T Moyo-Gwete",
"doi-asserted-by": "publisher",
"first-page": "2161",
"issue": "22",
"journal-title": "N. Engl. J. Med.",
"key": "6221_CR22",
"unstructured": "Moyo-Gwete, T. et al. Cross-reactive neutralizing antibody responses elicited by SARS-CoV-2 501Y.V2 (B.1.351). N. Engl. J. Med. 384(22), 2161–2163 (2021).",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2031304",
"author": "VA Simonovich",
"doi-asserted-by": "publisher",
"first-page": "619",
"issue": "7",
"journal-title": "N. Engl. J. Med.",
"key": "6221_CR23",
"unstructured": "Simonovich, V. A. et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N. Engl. J. Med. 384(7), 619–629 (2021).",
"volume": "384",
"year": "2021"
},
{
"key": "6221_CR24",
"unstructured": "US Department of Health and Human Services Food and Drug Administration. Letter of Authorization, Reissuance of Convalescent Plasma EUA. 2021. https://www.fda.gov/media/141477/download (accessed 5 June 2021)."
},
{
"key": "6221_CR25",
"unstructured": "World Health Organization. WHO R&D Blueprint: COVID-19 Therapeutic Trial Synopsis. Geneva, 2020. http://www10.who.int/blueprint/priority-diseases/key-action/COVID-19_Treatment_Trial_Design_Master_Protocol_synopsis_Final_18022020.pdfp6. (accessed 13 May 2020)."
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"author": "F Zhou",
"doi-asserted-by": "publisher",
"first-page": "1054",
"issue": "10229",
"journal-title": "Lancet",
"key": "6221_CR26",
"unstructured": "Zhou, F. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 395(10229), 1054–1062 (2020).",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2002032",
"author": "WJ Guan",
"doi-asserted-by": "publisher",
"first-page": "1708",
"issue": "18",
"journal-title": "N. Engl. J. Med.",
"key": "6221_CR27",
"unstructured": "Guan, W. J. et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 382(18), 1708–1720 (2020).",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"author": "C Huang",
"doi-asserted-by": "publisher",
"first-page": "497",
"issue": "10223",
"journal-title": "Lancet",
"key": "6221_CR28",
"unstructured": "Huang, C. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395(10223), 497–506 (2020).",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1038/s41591-021-01285-x",
"author": "CK Wibmer",
"doi-asserted-by": "publisher",
"first-page": "622",
"issue": "4",
"journal-title": "Nat. Med.",
"key": "6221_CR29",
"unstructured": "Wibmer, C. K. et al. SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma. Nat. Med. 27(4), 622–625 (2021).",
"volume": "27",
"year": "2021"
},
{
"key": "6221_CR30",
"unstructured": "Centers for Disease Control and Prevention Defining Adult Overweight & Obesity. https://www.cdc.gov/obesity/adult/defining.html (accessed 23 July 2021)."
},
{
"DOI": "10.1136/bmj.m3939",
"author": "A Agarwal",
"doi-asserted-by": "publisher",
"first-page": "m3939",
"journal-title": "BMJ",
"key": "6221_CR31",
"unstructured": "Agarwal, A. et al. Convalescent plasma in the management of moderate covid-19 in adults in India: Open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ 371, m3939 (2020).",
"volume": "371",
"year": "2020"
},
{
"DOI": "10.1038/s41586-021-03402-9",
"author": "H Tegally",
"doi-asserted-by": "publisher",
"first-page": "438",
"issue": "7854",
"journal-title": "Nature",
"key": "6221_CR32",
"unstructured": "Tegally, H. et al. Detection of a SARS-CoV-2 variant of concern in South Africa. Nature 592(7854), 438–443 (2021).",
"volume": "592",
"year": "2021"
},
{
"DOI": "10.1016/j.cell.2021.02.037",
"author": "D Zhou",
"doi-asserted-by": "publisher",
"first-page": "2348",
"issue": "9",
"journal-title": "Cell",
"key": "6221_CR33",
"unstructured": "Zhou, D. et al. Evidence of escape of SARS-CoV-2 variant B.1.351 from natural and vaccine-induced sera. Cell 184(9), 2348–61 e6 (2021).",
"volume": "184",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2033700",
"author": "R Libster",
"doi-asserted-by": "publisher",
"first-page": "610",
"issue": "7",
"journal-title": "N. Engl. J. Med.",
"key": "6221_CR34",
"unstructured": "Libster, R. et al. Early high-titer plasma therapy to prevent severe Covid-19 in older adults. N. Engl. J. Med. 384(7), 610–618 (2021).",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1101/2021.02.02",
"doi-asserted-by": "publisher",
"key": "6221_CR35",
"unstructured": "González, S. E., Regairaz, L., Salazar, M., et al. Timing of convalescent plasma administration and 28-day mortality for COVID-19 pneumonia. medRxiv 2021: 21250758 February 2, 2021. (Accessed 12 Aug 2021). https://doi.org/10.1101/2021.02.02."
},
{
"DOI": "10.1056/NEJMoa2102685",
"author": "M Dougan",
"doi-asserted-by": "publisher",
"journal-title": "N. Engl. J. Med.",
"key": "6221_CR36",
"unstructured": "Dougan, M. et al. Bamlanivimab plus Etesevimab in Mild or Moderate Covid-19. N. Engl. J. Med. https://doi.org/10.1056/NEJMoa2102685 (2021).",
"year": "2021"
},
{
"key": "6221_CR37",
"unstructured": "Infectious Diseases Society of America. Clarifying the Emergency Use Authorization framework for COVID-19 convalescent plasma: considerations for clinicians prepared jointly by the Infectious Diseases Society of America and AABB. November 18, 2020. https://www.idsociety.org/globalassets/covid-19-real-time-learning-network/therapeutics-and-interventions/convalescent-plasma/aabb-idsa-convalescent-plasma-eua--final.pdf. (Accessed 31 Aug 2021)."
},
{
"DOI": "10.1056/NEJMoa2103784",
"author": "FK Korley",
"doi-asserted-by": "publisher",
"first-page": "1951",
"journal-title": "N. Engl. J. Med.",
"key": "6221_CR38",
"unstructured": "Korley, F. K. et al. Early convalescent plasma for high-risk outpatients with Covid-19. N. Engl. J. Med. 385, 1951–1960 (2021).",
"volume": "385",
"year": "2021"
},
{
"key": "6221_CR39",
"unstructured": "Ling, R. R., Sim, J. J. L., Tan, F. L., et al. Convalescent plasma for patients hospitalized with coronavirus disease 2019: A meta-analysis with trial sequential analysis of randomized controlled trials. Transfus Med Rev. S0887-7963(21)00056-0 (2021)."
},
{
"DOI": "10.1186/s12879-021-06829-7",
"author": "C Axfors",
"doi-asserted-by": "publisher",
"first-page": "1170",
"issue": "1",
"journal-title": "BMC Infect. Dis.",
"key": "6221_CR40",
"unstructured": "Axfors, C. et al. Association between convalescent plasma treatment and mortality in COVID-19: A collaborative systematic review and meta-analysis of randomized clinical trials. BMC Infect. Dis. 21(1), 1170 (2021).",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2033130",
"author": "JD Lundgren",
"doi-asserted-by": "publisher",
"first-page": "905",
"issue": "10",
"journal-title": "N. Engl. J. Med.",
"key": "6221_CR41",
"unstructured": "Lundgren, J. D. et al. On behalf of the Activ-Tico Ly- CoV555 Study Group. A neutralizing monoclonal antibody for hospitalized patients with Covid-19. N. Engl. J. Med. 384(10), 905–914 (2021).",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1101/2020.11.08",
"author": "JW Senefeld",
"doi-asserted-by": "publisher",
"journal-title": "medRxiv",
"key": "6221_CR42",
"unstructured": "Senefeld, J. W. et al. Therapeutic use of convalescent plasma in COVID-19 patients with immunodeficiency. medRxiv https://doi.org/10.1101/2020.11.08 (2020).",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa1198",
"author": "Western Cape Department of Health in collaboration with the National Institute for Communicable Diseases SA. Risk Factors for Coronavirus Disease",
"doi-asserted-by": "publisher",
"journal-title": "Clin. Infect. Dis.",
"key": "6221_CR43",
"unstructured": "Western Cape Department of Health in collaboration with the National Institute for Communicable Diseases SA. Risk Factors for Coronavirus Disease. (COVID-19) death in a population cohort study from the Western Cape Province, South Africa. Clin. Infect. Dis. https://doi.org/10.1093/cid/ciaa1198 (2019).",
"year": "2019"
}
],
"reference-count": 43,
"references-count": 43,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.nature.com/articles/s41598-022-06221-8"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Multidisciplinary"
],
"subtitle": [],
"title": "Convalescent plasma in the treatment of moderate to severe COVID-19 pneumonia: a randomized controlled trial (PROTECT-Patient Trial)",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "12"
}