Effects of Losartan on Patients Hospitalized for Acute COVID-19: A Randomized Controlled Trial
et al., Clinical Infectious Diseases, doi:10.1093/cid/ciae306, ARBs CORONA II, NCT04606563, Jul 2024
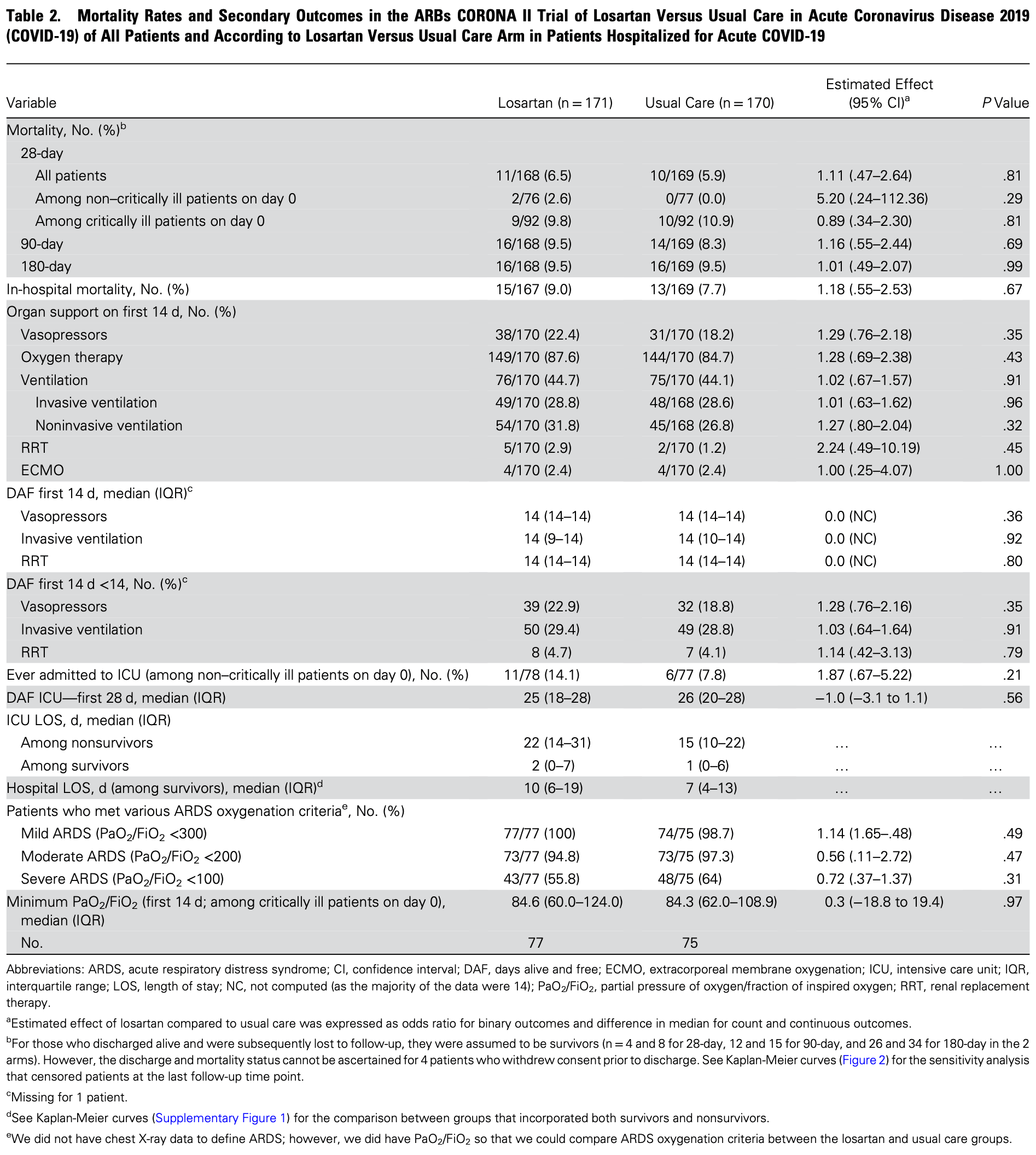
RCT 341 hospitalized COVID-19 patients showing significant harm with losartan treatment. The trial was stopped early due to safety concerns after losartan patients experienced significantly higher rates of serious adverse events (39.8% vs 27.2%) and hypotension (30.4% vs 15.3%) compared to usual care, with no mortality benefit.
|
risk of death, 14.3% higher, RR 1.14, p = 0.69, treatment 16 of 171 (9.4%), control 16 of 170 (9.4%), odds ratio converted to relative risk, day 90.
|
|
risk of death, 10.3% higher, RR 1.10, p = 0.81, treatment 11 of 171 (6.4%), control 10 of 170 (5.9%), odds ratio converted to relative risk, day 28.
|
|
risk of mechanical ventilation, 0.7% higher, RR 1.01, p = 0.96, treatment 49 of 170 (28.8%), control 48 of 168 (28.6%), odds ratio converted to relative risk, day 14.
|
|
risk of oxygen therapy, 3.5% higher, RR 1.03, p = 0.43, treatment 149 of 170 (87.6%), control 144 of 170 (84.7%), odds ratio converted to relative risk, day 14.
|
|
risk of ICU admission, 75.1% higher, RR 1.75, p = 0.21, treatment 11 of 78 (14.1%), control 6 of 77 (7.8%), odds ratio converted to relative risk.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Tran et al., 10 Jul 2024, Randomized Controlled Trial, multiple countries, peer-reviewed, mean age 56.4, 102 authors, study period 20 October, 2020 - 1 March, 2022, trial NCT04606563 (history) (ARBs CORONA II).
Contact: jim.russell@hli.ubc.ca.
Effects of Losartan on Patients Hospitalized for Acute COVID-19: A Randomized Controlled Trial
Clinical Infectious Diseases, doi:10.1093/cid/ciae306
Background. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) down-regulates angiotensin-converting enzyme 2, potentially increasing angiotensin II. We hypothesized that losartan compared to usual care decreases mortality and is safe in patients hospitalized with coronavirus disease 2019 . We aimed to evaluate the effect of losartan versus usual care on 28-day mortality in patients hospitalized for acute COVID-19. Methods. Eligibility criteria included adults admitted for acute COVID-19. Exclusion criteria were hypotension, hyperkalemia, acute kidney injury, and use of angiotensin receptor blockers (ARBs) or angiotensin-converting enzyme inhibitors within 7 days. Participants were randomized to losartan 25-100 mg/day orally for the hospital duration or 3 months or the control arm (usual care) in 29 hospitals in Canada and France. The primary outcome was 28-day mortality. Secondary outcomes were hospital mortality, organ support, and serious adverse events (SAEs). Results. The trial was stopped early because of a serious safety concern with losartan. In 341 patients, any SAE and hypotension were significantly higher in the losartan versus usual care groups (any SAE: 39.8% vs 27.2%, respectively, P = .01; hypotension: 30.4% vs 15.3%, respectively, P < .001) in both ward and intensive care patients. The 28-day mortality did not differ between losartan (6.5%) versus usual care (5.9%) (odds ratio, 1.11 [95% confidence interval, .47-2.64]; P = .81), nor did organ dysfunction or secondary outcomes. Conclusions. Caution is needed in deciding which patients to start or continue using ARBs in patients hospitalized with pneumonia to mitigate risk of hypotension, acute kidney injury, and other side effects. ARBs should not be added to care of patients hospitalized for acute COVID-19. Clinical Trials Registration. NCT04606563.
Supplementary Data Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes Author contributions. Conception and design: J. A. R. and K. C. T. Analysis: T. L. and J. S. Interpretation: All authors. Drafting the manuscript for important intellectual content: All authors. Acknowledgments. The authors thank the patients and their families for giving permission to include them and to report this trial. They also thank the many caregivers who worked tirelessly through the coronavirus disease 2019 (COVID-19) pandemic to provide care to the patients included herein. They also thank the funding agencies. Financial support. This work was supported by the Canadian Institutes of Health Research (CIHR) (grant numbers RN420682 to J. A. R.). Potential conflicts of interest. J. A. R. reports patents owned by the University of British Columbia that are related to (i) the use of PCSK9 inhibitor(s) in sepsis, (ii) the use of vasopressin in septic shock, and (iii) a patent owned by Ferring for use of selepressin in septic shock; J. A. R. is an inventor on these patents. J. A. R. was a founder, director, and shareholder in Cyon Therapeutics Inc (now closed) and is a shareholder in Molecular You Corp; is the Senior Research Advisor of the..
References
Bauer, Schreinlechner, Sappler, Discontinuation versus continuation of renin-angiotensin-system inhibitors in COVID-19 (ACEI-COVID): a prospective, parallel group, randomised, controlled, open-label trial, Lancet Respir Med
Cohen, Hanff, William, Continuation versus discontinuation of renin-angiotensin system inhibitors in patients admitted to hospital with COVID-19: a prospective, randomised, open-label trial, Lancet Respir Med
D'elia, Bayliss, Gleason, Weinrauch, Cardiovascular-renal complications and the possible role of plasminogen activator inhibitor: a review, Clin Kidney J
Dominiczak, Mancia, Joint editorial for the International Society of Hypertension guidelines, Hypertension
Duarte, Pelorosso, Nicolosi, Telmisartan for treatment of Covid-19 patients: an open multicenter randomized clinical trial, EClinicalMedicine
Ferritin, None, median
Files, Gibbs, Schaich, A pilot study to assess the circulating renin-angiotensin system in COVID-19 acute respiratory failure, Am J Physiol Lung Cell Mol Physiol
Geriak, Haddad, Kullar, Randomized prospective open label study shows no impact on clinical outcome of adding losartan to hospitalized COVID-19 patients with mild hypoxemia, Infect Dis Ther
Gnanenthiran, Borghi, Burger, Renin-angiotensin system inhibitors in patients with COVID-19: a meta-analysis of randomized controlled trials led by the International Society of Hypertension, J Am Heart Assoc
Group; Horby, Lim, Emberson, Dexamethasone in hospitalized patients with Covid-19, N Engl J Med
Hemoglobin, ) 135, Platelet count
Hoffmann, Kleine-Weber, Schroeder, SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell
Ibrahim, Jiroutek, Holland, Sutton, Utilization of angiotensin converting enzyme inhibitors (ACEI) and angiotensin receptor blockers (ARB) in patients diagnosed with diabetes: analysis from the National Ambulatory Medical Care Survey, Prev Med Rep
Imai, Kuba, Rao, Angiotensin-converting enzyme 2 protects from severe acute lung failure, Nature
Investigators, Lawler, Derde, Van De Veerdonk, Effect of angiotensin-converting enzyme inhibitor and angiotensin receptor blocker initiation on organ support-free days in patients hospitalized with COVID-19: a randomized clinical trial, JAMA
Johansen, Yun, Griggs, Jackson, Richardson, Anti-hypertensive medication combinations in the United States, J Am Board Fam Med
Kalil, Patterson, Mehta, Baricitinib plus remdesivir for hospitalized adults with Covid-19, N Engl J Med
Knight, Ho, Pius, Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: development and validation of the 4C mortality score, BMJ
Koh, Chung, Ahn, Angiotensin II type 1 receptor blockers reduce tissue factor activity and plasminogen activator inhibitor type-1 antigen in hypertensive patients: a randomized, double-blind, placebo-controlled study, Atherosclerosis
Lee, Cau, Cheng, Angiotensin receptor blockers and angiotensinconverting enzyme inhibitors in COVID-19: meta-analysis/meta-regression adjusted for confounding factors, CJC Open
Lee, Cheng, Vinh, Organ dysfunction and death in patients admitted to hospital with COVID-19 in pandemic waves 1 to 3 in British Columbia, Ontario and Quebec, Canada: a cohort study, CMAJ Open
Lee, Kondo, Campbell, Effects of renin-angiotensin system blockers on outcomes from COVID-19: a systematic review and meta-analysis of randomized controlled trials, Eur Heart J Cardiovasc Pharmacother
Liu, Yang, Zhang, Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury, Sci China Life Sci
Lopes, Macedo, De, Silva, Effect of discontinuing vs continuing angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on days alive and out of the hospital in patients admitted with COVID-19: a randomized clinical trial, JAMA
Mehta, Griendling, Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system, Am J Physiol Cell Physiol
Montiel, Lobysheva, Gérard, Oxidative stress-induced endothelial dysfunction and decreased vascular nitric oxide in COVID-19 patients, EBioMedicine
Murphy, Drawz, Foley, Trends in angiotensin-converting enzyme inhibitor and angiotensin II receptor blocker use among those with impaired kidney function in the United States, J Am Soc Nephrol
Nouri-Vaskeh, Kalami, Zand, Comparison of losartan and amlodipine effects on the outcomes of patient with COVID-19 and primary hypertension: a randomised clinical trial, Int J Clin Pract
Puskarich, Ingraham, Merck, Efficacy of losartan in hospitalized patients with COVID-19-induced lung injury: a randomized clinical trial, JAMA Netw Open
Rocheleau, Lee, Mohammed, Renin-angiotensin system pathway therapeutics associated with improved outcomes in males hospitalized with COVID-19, Crit Care Med
Stefano, Ram, Scharfstein, Losartan in hospitalized patients with COVID-19 in North America: an individual participant data meta-analysis, Medicine
Tran, Aldemerdash, Chang, Guideline-directed medical therapy and survival following hospitalization in patients with heart failure, Pharmacotherapy
Vaduganathan, Vardeny, Michel, Mcmurray, Pfeffer et al., Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19, N Engl J Med
Wang, Gheblawi, Nikhanj, Dysregulation of ACE (angiotensinconverting enzyme)-2 and renin-angiotensin peptides in SARS-CoV-2 mediated mortality and end-organ injuries, Hypertension
Yang, Gu, Zhao, Angiotensin-converting enzyme 2 (ACE2) mediates influenza H7N9 virus-induced acute lung injury, Sci Rep
Yin, Huang, Li, Tang, Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2, J Thromb Thrombolysis
DOI record:
{
"DOI": "10.1093/cid/ciae306",
"ISSN": [
"1058-4838",
"1537-6591"
],
"URL": "http://dx.doi.org/10.1093/cid/ciae306",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) down-regulates angiotensin-converting enzyme 2, potentially increasing angiotensin II. We hypothesized that losartan compared to usual care decreases mortality and is safe in patients hospitalized with coronavirus disease 2019 (COVID-19). We aimed to evaluate the effect of losartan versus usual care on 28-day mortality in patients hospitalized for acute COVID-19.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>Eligibility criteria included adults admitted for acute COVID-19. Exclusion criteria were hypotension, hyperkalemia, acute kidney injury, and use of angiotensin receptor blockers (ARBs) or angiotensin-converting enzyme inhibitors within 7 days. Participants were randomized to losartan 25–100 mg/day orally for the hospital duration or 3 months or the control arm (usual care) in 29 hospitals in Canada and France. The primary outcome was 28-day mortality. Secondary outcomes were hospital mortality, organ support, and serious adverse events (SAEs).</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>The trial was stopped early because of a serious safety concern with losartan. In 341 patients, any SAE and hypotension were significantly higher in the losartan versus usual care groups (any SAE: 39.8% vs 27.2%, respectively, P = .01; hypotension: 30.4% vs 15.3%, respectively, P &lt; .001) in both ward and intensive care patients. The 28-day mortality did not differ between losartan (6.5%) versus usual care (5.9%) (odds ratio, 1.11 [95% confidence interval, .47–2.64]; P = .81), nor did organ dysfunction or secondary outcomes.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>Caution is needed in deciding which patients to start or continue using ARBs in patients hospitalized with pneumonia to mitigate risk of hypotension, acute kidney injury, and other side effects. ARBs should not be added to care of patients hospitalized for acute COVID-19.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Clinical Trials Registration</jats:title>\n <jats:p>NCT04606563.</jats:p>\n </jats:sec>",
"author": [
{
"affiliation": [
{
"name": "Division of General Internal Medicine, Vancouver General Hospital, University of British Columbia , Vancouver ,",
"place": [
"Canada"
]
}
],
"family": "Tran",
"given": "Karen C",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Service de Médecine Intensive-Réanimation, Centre Hospitalier Universitaire d’Angers , Angers ,",
"place": [
"France"
]
}
],
"family": "Asfar",
"given": "Pierre",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "McGill’s Interdisciplinary Initiative in Infection and Immunity, Divisions of Infectious Diseases and Medical Microbiology, McGill University Health Centre , Montreal, Quebec ,",
"place": [
"Canada"
]
}
],
"family": "Cheng",
"given": "Matthew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Service de Médecine Intensive-Réanimation, Nouvel Hôpital Civil, Hôpitaux Universitaires de Strasbourg , Strasbourg ,",
"place": [
"France"
]
}
],
"family": "Demiselle",
"given": "Julien",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Centre for Health Evaluation and Outcome Science, St Paul's Hospital and University of British Columbia , Vancouver ,",
"place": [
"Canada"
]
}
],
"family": "Singer",
"given": "Joel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Centre for Health Evaluation and Outcome Science, St Paul's Hospital and University of British Columbia , Vancouver ,",
"place": [
"Canada"
]
}
],
"family": "Lee",
"given": "Terry",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of General Internal Medicine, Vancouver General Hospital, University of British Columbia , Vancouver ,",
"place": [
"Canada"
]
}
],
"family": "Sweet",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Critical Care Medicine, and Centre for Heart Lung Innovation, St Paul's Hospital , Vancouver ,",
"place": [
"Canada"
]
}
],
"family": "Boyd",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Critical Care Medicine, and Centre for Heart Lung Innovation, St Paul's Hospital , Vancouver ,",
"place": [
"Canada"
]
}
],
"family": "Walley",
"given": "Keith",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine and Critical Care Medicine, Surrey Memorial Hospital , British Columbia ,",
"place": [
"Canada"
]
}
],
"family": "Haljan",
"given": "Greg",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine and Critical Care Medicine, Surrey Memorial Hospital , British Columbia ,",
"place": [
"Canada"
]
}
],
"family": "Sharif",
"given": "Omar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Service de Médecine Intensive-Réanimation, Assistance Publique–Hôpitaux de Paris Ambroise Paré , Boulogne ,",
"place": [
"France"
]
}
],
"family": "Geri",
"given": "Guillaume",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Service de Réanimation Polyvalente, Centre Hospitalier de Cholet"
}
],
"family": "Auchabie",
"given": "Johann",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Service de Médecine Intensive-Réanimation, Centre Hospitalier Universitaire Dijon , Dijon ,",
"place": [
"France"
]
}
],
"family": "Quenot",
"given": "Jean-Pierre",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "McGill's Interdisciplinary Initiative in Infection and Immunity, McGill University Health Centre , Montreal, Quebec ,",
"place": [
"Canada"
]
}
],
"family": "Lee",
"given": "Todd C",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Niagara Health, McMaster University , St Catherines, Ontario ,",
"place": [
"Canada"
]
}
],
"family": "Tsang",
"given": "Jennifer",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Service de Médecine Intensive-Réanimation, Nouvel Hôpital Civil Strasbourg , Strasbourg ,",
"place": [
"France"
]
}
],
"family": "Meziani",
"given": "Ferhat",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Centre Hospitalier Universitaire de Sherbrooke, University of Sherbrooke , Quebec ,",
"place": [
"Canada"
]
}
],
"family": "Lamontagne",
"given": "Francois",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Service de Maladies Infectieuses, Centre Hospitalier Universitaire d'Angers , Angers ,",
"place": [
"France"
]
}
],
"family": "Dubee",
"given": "Vincent",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Service de Réanimation Chirurgicale, Centre Hospitalier Universitaire Angers , Angers ,",
"place": [
"France"
]
}
],
"family": "Lasocki",
"given": "Sigismond",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Royal Jubilee Hospital, Island Health , Victoria, British Columbia"
}
],
"family": "Ovakim",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Royal Jubilee Hospital, Island Health , Victoria, British Columbia"
}
],
"family": "Wood",
"given": "Gordon",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Centre Hospitalier Universitaire de Québec–Université Laval , Quebec ,",
"place": [
"Canada"
]
}
],
"family": "Turgeon",
"given": "Alexis",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Service de Médecine Intensive-Réanimation, Assistance Publique–Hôpitaux de Paris Avicenne , Bobigny ,",
"place": [
"France"
]
}
],
"family": "Cohen",
"given": "Yves",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Service de Réanimation Polyvalente, Centre Hospitalier Bretagne-Atlantique , Vannes ,",
"place": [
"France"
]
}
],
"family": "Lebas",
"given": "Eddy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Service de Réanimation Polyvalente, Centre Hospitalier Universitaire Limoges , Limoges ,",
"place": [
"France"
]
}
],
"family": "Goudelin",
"given": "Marine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Nanaimo Regional General Hospital , British Columbia ,",
"place": [
"Canada"
]
}
],
"family": "Forrest",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Nanaimo Regional General Hospital , British Columbia ,",
"place": [
"Canada"
]
}
],
"family": "Teale",
"given": "Alastair",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Service de Médecine Intensive-Réanimation, Assistance Publique-Hôpitaux de Paris , Cochin ,",
"place": [
"France"
]
}
],
"family": "Mira",
"given": "Jean-Paul",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Critical Care Medicine, Sunnybrook Health Sciences Centre , Toronto, Ontario ,",
"place": [
"Canada"
]
}
],
"family": "Fowler",
"given": "Robert",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Critical Care Medicine, Sunnybrook Health Sciences Centre , Toronto, Ontario ,",
"place": [
"Canada"
]
}
],
"family": "Daneman",
"given": "Nick",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Critical Care Medicine, Sunnybrook Health Sciences Centre , Toronto, Ontario ,",
"place": [
"Canada"
]
}
],
"family": "Adhikari",
"given": "Neill K J",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Service de Médecine Interne–Maladies Infectieuses–Hématologie, Centre Hospitalier Bretagne-Atlantique , Vannes ,",
"place": [
"France"
]
}
],
"family": "Gousseff",
"given": "Marie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Service de médecine polyvalente et maladies infectieuses, Centre Hospitalier Melun , Melun ,",
"place": [
"France"
]
}
],
"family": "Leroy",
"given": "Pierre",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Service de Réanimation Polyvalente, Centre Hospitalier Argenteuil ,",
"place": [
"France"
]
}
],
"family": "Plantefeve",
"given": "Gaetan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Service de médecine interne, Centre Hospitalier Agen , Agen ,",
"place": [
"France"
]
}
],
"family": "Rispal",
"given": "Patrick",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Service de Médecine post-urgences–Maladies infectieuses, Centre Hospitalier de Cholet , Cholet ,",
"place": [
"France"
]
}
],
"family": "Courtois",
"given": "Roxane",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Departments of Critical Care Medicine, Medicine, and Biochemistry and Molecular Biology, Foothills Medical Centre, University of Calgary , Alberta ,",
"place": [
"Canada"
]
}
],
"family": "Winston",
"given": "Brent",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Critical Care Medicine, Royal Columbian Hospital , New Westminster, British Columbia ,",
"place": [
"Canada"
]
},
{
"name": "Department of Medicine, Simon Fraser University , Surrey, British Columbia ,",
"place": [
"Canada"
]
}
],
"family": "Reynolds",
"given": "Steve",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Critical Care Medicine, Royal Columbian Hospital , New Westminster, British Columbia ,",
"place": [
"Canada"
]
},
{
"name": "Department of Medicine, Simon Fraser University , Surrey, British Columbia ,",
"place": [
"Canada"
]
}
],
"family": "Birks",
"given": "Peter",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Service de médecine interne, Hôpital St Joseph , Marseille ,",
"place": [
"France"
]
}
],
"family": "Bienvenu",
"given": "Boris",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Service de Médecine Intensive-Réanimation et de Maladies Infectieuses, Centre Hospitalier Universitaire de Rennes , Rennes ,",
"place": [
"France"
]
}
],
"family": "Tadie",
"given": "Jean-Marc",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Service de médecine interne, maladies du sang et infectiologie, Centre Hospitalier de Quimper , Quimper ,",
"place": [
"France"
]
}
],
"family": "Talarmin",
"given": "Jean-Philippe",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Service de Maladies Infectieuses, Centre Hospitalier Régional Universitaire Brest , Brest ,",
"place": [
"France"
]
}
],
"family": "Ansart",
"given": "Severine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Critical Care Medicine, and Centre for Heart Lung Innovation, St Paul's Hospital , Vancouver ,",
"place": [
"Canada"
]
}
],
"family": "Russell",
"given": "James A",
"sequence": "additional"
},
{
"affiliation": [],
"name": "for the ARBs CORONA II Team",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Russell",
"given": "J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tran",
"given": "K",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cheng",
"given": "M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Asfar",
"given": "P",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Demiselle",
"given": "J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Singer",
"given": "J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mann",
"given": "P",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jain",
"given": "F",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tran",
"given": "K",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Donohoe",
"given": "K",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Leung",
"given": "V",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lee",
"given": "T",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tran",
"given": "K",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Boyd",
"given": "J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Walley",
"given": "K",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tran",
"given": "K",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sweet",
"given": "D",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Haljan",
"given": "G",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sharif",
"given": "O",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ovakim",
"given": "D",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wood",
"given": "G",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Forrest",
"given": "D",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Teale",
"given": "A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Reynolds",
"given": "S",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Birk",
"given": "P",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Winston",
"given": "B",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fowler",
"given": "R",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dameman",
"given": "N",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Adhikari",
"given": "N",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tsang",
"given": "J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cheng",
"given": "M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lamontagne",
"given": "F",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Turgeon-Fournier",
"given": "A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Asfar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Demiselle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Geri",
"given": "D G",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Auchabie",
"given": "J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Quenot",
"given": "J P",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Meziani",
"given": "F",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dubee",
"given": "V",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lasocki",
"given": "S",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cohen",
"given": "Y",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lebas",
"given": "E",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Goudelin",
"given": "M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mira",
"given": "J P",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gousseff",
"given": "M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Leroy",
"given": "P",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Plantefev",
"given": "G",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rispal",
"given": "P",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Courtois",
"given": "R",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bievenue",
"given": "B",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tadie",
"given": "J M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Talarmin",
"given": "J P",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ansart",
"given": "S",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yi",
"given": "Tae Won",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Levin",
"given": "Adeera",
"sequence": "additional"
}
],
"container-title": "Clinical Infectious Diseases",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
7,
10
]
],
"date-time": "2024-07-10T15:17:39Z",
"timestamp": 1720624659000
},
"deposited": {
"date-parts": [
[
2024,
9,
26
]
],
"date-time": "2024-09-26T16:04:23Z",
"timestamp": 1727366663000
},
"funder": [
{
"DOI": "10.13039/501100000024",
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100000024",
"id-type": "DOI"
}
],
"name": "Canadian Institutes of Health Research"
}
],
"indexed": {
"date-parts": [
[
2025,
11,
5
]
],
"date-time": "2025-11-05T21:19:31Z",
"timestamp": 1762377571067,
"version": "3.37.3"
},
"is-referenced-by-count": 3,
"issue": "3",
"issued": {
"date-parts": [
[
2024,
7,
10
]
]
},
"journal-issue": {
"issue": "3",
"published-online": {
"date-parts": [
[
2024,
7,
10
]
]
},
"published-print": {
"date-parts": [
[
2024,
9,
26
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
7,
10
]
],
"date-time": "2024-07-10T00:00:00Z",
"timestamp": 1720569600000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/cid/advance-article-pdf/doi/10.1093/cid/ciae306/58447468/ciae306.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/cid/article-pdf/79/3/615/59357138/ciae306.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/cid/article-pdf/79/3/615/59357138/ciae306.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"page": "615-625",
"prefix": "10.1093",
"published": {
"date-parts": [
[
2024,
7,
10
]
]
},
"published-online": {
"date-parts": [
[
2024,
7,
10
]
]
},
"published-other": {
"date-parts": [
[
2024,
9,
15
]
]
},
"published-print": {
"date-parts": [
[
2024,
9,
26
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference": [
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in hospitalized patients with Covid-19",
"author": "RECOVERY Collaborative Group",
"doi-asserted-by": "crossref",
"first-page": "693",
"journal-title": "N Engl J Med",
"key": "2024092615382025600_ciae306-B1",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2105911",
"article-title": "Therapeutic anticoagulation with heparin in noncritically ill patients with Covid-19",
"author": "ATTACC Investigators",
"doi-asserted-by": "crossref",
"first-page": "790",
"journal-title": "N Engl J Med",
"key": "2024092615382025600_ciae306-B2",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(21)00676-0",
"article-title": "Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial",
"author": "RECOVERY Collaborative Group",
"doi-asserted-by": "crossref",
"first-page": "1637",
"journal-title": "Lancet",
"key": "2024092615382025600_ciae306-B3",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2031994",
"article-title": "Baricitinib plus remdesivir for hospitalized adults with Covid-19",
"author": "Kalil",
"doi-asserted-by": "crossref",
"first-page": "795",
"journal-title": "N Engl J Med",
"key": "2024092615382025600_ciae306-B4",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"article-title": "SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor",
"author": "Hoffmann",
"doi-asserted-by": "crossref",
"first-page": "271",
"journal-title": "Cell",
"key": "2024092615382025600_ciae306-B5",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1007/s11427-020-1643-8",
"article-title": "Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "364",
"journal-title": "Sci China Life Sci",
"key": "2024092615382025600_ciae306-B6",
"volume": "63",
"year": "2020"
},
{
"DOI": "10.1038/nature03712",
"article-title": "Angiotensin-converting enzyme 2 protects from severe acute lung failure",
"author": "Imai",
"doi-asserted-by": "crossref",
"first-page": "112",
"journal-title": "Nature",
"key": "2024092615382025600_ciae306-B7",
"volume": "436",
"year": "2005"
},
{
"DOI": "10.1007/s11239-020-02105-8",
"article-title": "Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2",
"author": "Yin",
"doi-asserted-by": "crossref",
"first-page": "1107",
"journal-title": "J Thromb Thrombolysis",
"key": "2024092615382025600_ciae306-B8",
"volume": "51",
"year": "2021"
},
{
"DOI": "10.1152/ajpcell.00287.2006",
"article-title": "Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system",
"author": "Mehta",
"doi-asserted-by": "crossref",
"first-page": "C82",
"journal-title": "Am J Physiol Cell Physiol",
"key": "2024092615382025600_ciae306-B9",
"volume": "292",
"year": "2007"
},
{
"DOI": "10.1093/ckj/sfw080",
"article-title": "Cardiovascular-renal complications and the possible role of plasminogen activator inhibitor: a review",
"author": "D’Elia",
"doi-asserted-by": "crossref",
"first-page": "705",
"journal-title": "Clin Kidney J",
"key": "2024092615382025600_ciae306-B10",
"volume": "9",
"year": "2016"
},
{
"DOI": "10.1016/j.atherosclerosis.2004.07.008",
"article-title": "Angiotensin II type 1 receptor blockers reduce tissue factor activity and plasminogen activator inhibitor type-1 antigen in hypertensive patients: a randomized, double-blind, placebo-controlled study",
"author": "Koh",
"doi-asserted-by": "crossref",
"first-page": "155",
"journal-title": "Atherosclerosis",
"key": "2024092615382025600_ciae306-B11",
"volume": "177",
"year": "2004"
},
{
"DOI": "10.1056/NEJMsr2005760",
"article-title": "Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19",
"author": "Vaduganathan",
"doi-asserted-by": "crossref",
"first-page": "1653",
"journal-title": "N Engl J Med",
"key": "2024092615382025600_ciae306-B12",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1038/srep07027",
"article-title": "Angiotensin-converting enzyme 2 (ACE2) mediates influenza H7N9 virus-induced acute lung injury",
"author": "Yang",
"doi-asserted-by": "crossref",
"first-page": "7027",
"journal-title": "Sci Rep",
"key": "2024092615382025600_ciae306-B13",
"volume": "4",
"year": "2014"
},
{
"DOI": "10.1016/j.eclinm.2021.100962",
"article-title": "Telmisartan for treatment of Covid-19 patients: an open multicenter randomized clinical trial",
"author": "Duarte",
"doi-asserted-by": "crossref",
"first-page": "100962",
"journal-title": "EClinicalMedicine",
"key": "2024092615382025600_ciae306-B14",
"volume": "37",
"year": "2021"
},
{
"DOI": "10.1007/s40121-021-00453-3",
"article-title": "Randomized prospective open label study shows no impact on clinical outcome of adding losartan to hospitalized COVID-19 patients with mild hypoxemia",
"author": "Geriak",
"doi-asserted-by": "crossref",
"first-page": "1323",
"journal-title": "Infect Dis Ther",
"key": "2024092615382025600_ciae306-B15",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1111/ijcp.14124",
"article-title": "Comparison of losartan and amlodipine effects on the outcomes of patient with COVID-19 and primary hypertension: a randomised clinical trial",
"author": "Nouri-Vaskeh",
"doi-asserted-by": "crossref",
"first-page": "e14124",
"journal-title": "Int J Clin Pract",
"key": "2024092615382025600_ciae306-B16",
"volume": "75",
"year": "2021"
},
{
"DOI": "10.1001/jamanetworkopen.2022.2735",
"article-title": "Efficacy of losartan in hospitalized patients with COVID-19-induced lung injury: a randomized clinical trial",
"author": "Puskarich",
"doi-asserted-by": "crossref",
"first-page": "e222735",
"journal-title": "JAMA Netw Open",
"key": "2024092615382025600_ciae306-B17",
"volume": "5",
"year": "2022"
},
{
"DOI": "10.1016/S2213-2600(21)00214-9",
"article-title": "Discontinuation versus continuation of renin-angiotensin-system inhibitors in COVID-19 (ACEI-COVID): a prospective, parallel group, randomised, controlled, open-label trial",
"author": "Bauer",
"doi-asserted-by": "crossref",
"first-page": "863",
"journal-title": "Lancet Respir Med",
"key": "2024092615382025600_ciae306-B18",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(20)30558-0",
"article-title": "Continuation versus discontinuation of renin-angiotensin system inhibitors in patients admitted to hospital with COVID-19: a prospective, randomised, open-label trial",
"author": "Cohen",
"doi-asserted-by": "crossref",
"first-page": "275",
"journal-title": "Lancet Respir Med",
"key": "2024092615382025600_ciae306-B19",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.25864",
"article-title": "Effect of discontinuing vs continuing angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on days alive and out of the hospital in patients admitted with COVID-19: a randomized clinical trial",
"author": "Lopes",
"doi-asserted-by": "crossref",
"first-page": "254",
"journal-title": "JAMA",
"key": "2024092615382025600_ciae306-B20",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1161/JAHA.122.026143",
"article-title": "Renin-angiotensin system inhibitors in patients with COVID-19: a meta-analysis of randomized controlled trials led by the International Society of Hypertension",
"author": "Gnanenthiran",
"doi-asserted-by": "crossref",
"first-page": "e026143",
"journal-title": "J Am Heart Assoc",
"key": "2024092615382025600_ciae306-B21",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1016/j.cjco.2021.03.001",
"article-title": "Angiotensin receptor blockers and angiotensin-converting enzyme inhibitors in COVID-19: meta-analysis/meta-regression adjusted for confounding factors",
"author": "Lee",
"doi-asserted-by": "crossref",
"first-page": "965",
"journal-title": "CJC Open",
"key": "2024092615382025600_ciae306-B22",
"volume": "3",
"year": "2021"
},
{
"DOI": "10.1097/CCM.0000000000005589",
"article-title": "Renin-angiotensin system pathway therapeutics associated with improved outcomes in males hospitalized with COVID-19",
"author": "Rocheleau",
"doi-asserted-by": "crossref",
"first-page": "1306",
"journal-title": "Crit Care Med",
"key": "2024092615382025600_ciae306-B23",
"volume": "50",
"year": "2022"
},
{
"DOI": "10.1136/bmj.m3339",
"article-title": "Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: development and validation of the 4C mortality score",
"author": "Knight",
"doi-asserted-by": "crossref",
"first-page": "m3339",
"journal-title": "BMJ",
"key": "2024092615382025600_ciae306-B24",
"volume": "370",
"year": "2020"
},
{
"DOI": "10.1097/MD.0000000000033904",
"article-title": "Losartan in hospitalized patients with COVID-19 in North America: an individual participant data meta-analysis",
"author": "Di Stefano",
"doi-asserted-by": "crossref",
"first-page": "e33904",
"journal-title": "Medicine (Baltimore)",
"key": "2024092615382025600_ciae306-B25",
"volume": "102",
"year": "2023"
},
{
"DOI": "10.1093/ehjcvp/pvad067",
"article-title": "Effects of renin-angiotensin system blockers on outcomes from COVID-19: a systematic review and meta-analysis of randomized controlled trials",
"author": "Lee",
"doi-asserted-by": "crossref",
"first-page": "68",
"journal-title": "Eur Heart J Cardiovasc Pharmacother",
"key": "2024092615382025600_ciae306-B26",
"volume": "10",
"year": "2024"
},
{
"DOI": "10.1001/jama.2023.4480",
"article-title": "Effect of angiotensin-converting enzyme inhibitor and angiotensin receptor blocker initiation on organ support-free days in patients hospitalized with COVID-19: a randomized clinical trial",
"author": "Writing Committee for the R-CAP Investigators;",
"doi-asserted-by": "crossref",
"first-page": "1183",
"journal-title": "JAMA",
"key": "2024092615382025600_ciae306-B27",
"volume": "329",
"year": "2023"
},
{
"DOI": "10.3122/jabfm.2020.01.190134",
"article-title": "Anti-hypertensive medication combinations in the United States",
"author": "Johansen",
"doi-asserted-by": "crossref",
"first-page": "143",
"journal-title": "J Am Board Fam Med",
"key": "2024092615382025600_ciae306-B28",
"volume": "33",
"year": "2020"
},
{
"DOI": "10.1681/ASN.2018100971",
"article-title": "Trends in angiotensin-converting enzyme inhibitor and angiotensin II receptor blocker use among those with impaired kidney function in the United States",
"author": "Murphy",
"doi-asserted-by": "crossref",
"first-page": "1314",
"journal-title": "J Am Soc Nephrol",
"key": "2024092615382025600_ciae306-B29",
"volume": "30",
"year": "2019"
},
{
"DOI": "10.1016/j.pmedr.2016.01.005",
"article-title": "Utilization of angiotensin converting enzyme inhibitors (ACEI) and angiotensin receptor blockers (ARB) in patients diagnosed with diabetes: analysis from the National Ambulatory Medical Care Survey",
"author": "Ibrahim",
"doi-asserted-by": "crossref",
"first-page": "166",
"journal-title": "Prev Med Rep",
"key": "2024092615382025600_ciae306-B30",
"volume": "3",
"year": "2016"
},
{
"DOI": "10.1002/phar.2091",
"article-title": "Guideline-directed medical therapy and survival following hospitalization in patients with heart failure",
"author": "Tran",
"doi-asserted-by": "crossref",
"first-page": "406",
"journal-title": "Pharmacotherapy",
"key": "2024092615382025600_ciae306-B31",
"volume": "38",
"year": "2018"
},
{
"DOI": "10.9778/cmajo.20210216",
"article-title": "Organ dysfunction and death in patients admitted to hospital with COVID-19 in pandemic waves 1 to 3 in British Columbia, Ontario and Quebec, Canada: a cohort study",
"author": "Lee",
"doi-asserted-by": "crossref",
"first-page": "E379",
"journal-title": "CMAJ Open",
"key": "2024092615382025600_ciae306-B32",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1161/HYPERTENSIONAHA.121.18295",
"article-title": "Dysregulation of ACE (angiotensin-converting enzyme)-2 and renin-angiotensin peptides in SARS-CoV-2 mediated mortality and end-organ injuries",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "365",
"journal-title": "Hypertension",
"key": "2024092615382025600_ciae306-B33",
"volume": "79",
"year": "2022"
},
{
"DOI": "10.1152/ajplung.00129.2021",
"article-title": "A pilot study to assess the circulating renin-angiotensin system in COVID-19 acute respiratory failure",
"author": "Files",
"doi-asserted-by": "crossref",
"first-page": "L213",
"journal-title": "Am J Physiol Lung Cell Mol Physiol",
"key": "2024092615382025600_ciae306-B34",
"volume": "321",
"year": "2021"
},
{
"DOI": "10.1016/j.ebiom.2022.103893",
"article-title": "Oxidative stress-induced endothelial dysfunction and decreased vascular nitric oxide in COVID-19 patients",
"author": "Montiel",
"doi-asserted-by": "crossref",
"first-page": "103893",
"journal-title": "EBioMedicine",
"key": "2024092615382025600_ciae306-B35",
"volume": "77",
"year": "2022"
},
{
"DOI": "10.1161/HYPERTENSIONAHA.120.15224",
"article-title": "Joint editorial for the International Society of Hypertension guidelines",
"author": "Dominiczak",
"doi-asserted-by": "crossref",
"first-page": "1419",
"journal-title": "Hypertension",
"key": "2024092615382025600_ciae306-B36",
"volume": "75",
"year": "2020"
}
],
"reference-count": 36,
"references-count": 36,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/cid/article/79/3/615/7706430"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Effects of Losartan on Patients Hospitalized for Acute COVID-19: A Randomized Controlled Trial",
"type": "journal-article",
"volume": "79"
}