A Prospective Study of AFree Oral Spray as an Adjuvant Therapy for Mild and Moderate COVID-19 in Community Health Stations: Clinical Progression and Viral Clearance Outcomes
et al., In Vivo, doi:10.21873/invivo.13313, Aug 2023
Zinc for COVID-19
2nd treatment shown to reduce risk in
July 2020, now with p = 0.00000028 from 47 studies, recognized in 23 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 1,252 mild/moderate COVID-19 patients Vietnam, showing faster recovery and faster viral clearance with an oral spray containing zinc, propolis, xylitol, ginger, and DMSO. 5-10 times per day.
Targeted administration to the respiratory tract provides treatment directly
to the typical source of initial SARS-CoV-2 infection and replication, and
allows for rapid onset of action, higher local drug concentration, and reduced systemic side effects.
This study is excluded in meta-analysis:
many combined treatments which may significantly contribute to the effect seen.
Study covers propolis and zinc.
|
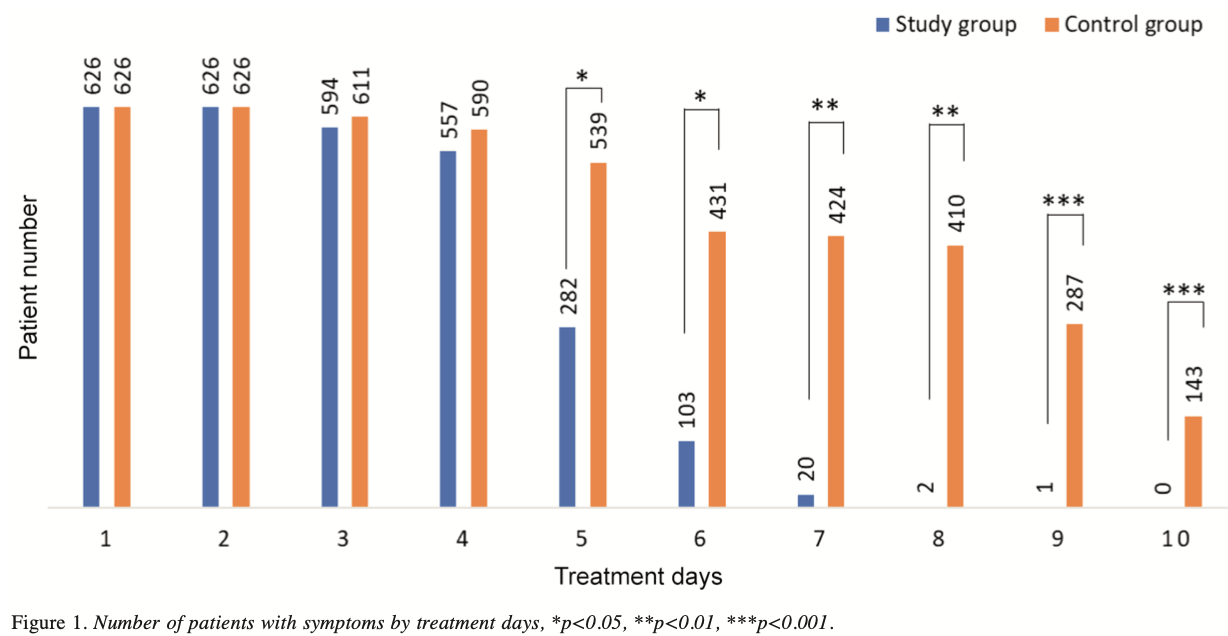
risk of no recovery, 99.7% lower, RR 0.003, p < 0.001, treatment 0 of 626 (0.0%), control 143 of 626 (22.8%), NNT 4.4, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), day 10.
|
|
risk of no recovery, 99.7% lower, RR 0.003, p < 0.001, treatment 1 of 626 (0.2%), control 287 of 626 (45.8%), NNT 2.2, day 9.
|
|
risk of no recovery, 99.5% lower, RR 0.005, p < 0.001, treatment 2 of 626 (0.3%), control 410 of 626 (65.5%), NNT 1.5, day 8.
|
|
risk of no recovery, 95.3% lower, RR 0.05, p < 0.001, treatment 20 of 626 (3.2%), control 424 of 626 (67.7%), NNT 1.5, day 7.
|
|
risk of no recovery, 76.1% lower, RR 0.24, p < 0.001, treatment 103 of 626 (16.5%), control 431 of 626 (68.8%), NNT 1.9, day 6.
|
|
risk of no recovery, 47.7% lower, RR 0.52, p < 0.001, treatment 282 of 626 (45.0%), control 539 of 626 (86.1%), NNT 2.4, day 5.
|
|
risk of no recovery, 5.6% lower, RR 0.94, p = 0.001, treatment 557 of 626 (89.0%), control 590 of 626 (94.2%), NNT 19, day 4.
|
|
risk of no recovery, 2.8% lower, RR 0.97, p = 0.02, treatment 594 of 626 (94.9%), control 611 of 626 (97.6%), NNT 37, day 3.
|
|
risk of no viral clearance, 97.8% lower, RR 0.02, p < 0.001, treatment 7 of 626 (1.1%), control 316 of 626 (50.5%), NNT 2.0, day 10.
|
|
risk of no viral clearance, 90.0% lower, RR 0.10, p < 0.001, treatment 58 of 626 (9.3%), control 582 of 626 (93.0%), NNT 1.2, day 7.
|
|
risk of no viral clearance, 16.2% lower, RR 0.84, p < 0.001, treatment 518 of 626 (82.7%), control 618 of 626 (98.7%), NNT 6.3, day 4.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Tran et al., 31 Aug 2023, Single Blind Randomized Controlled Trial, Vietnam, peer-reviewed, mean age 36.7, 7 authors, this trial uses multiple treatments in the treatment arm (combined with ginger, propolis, xylitol, and DMSO) - results of individual treatments may vary.
Contact: baxuanho@usc.edu.
Abstract: in vivo 37: 2155-2160 (2023)
doi:10.21873/invivo.13313
A Prospective Study of AFree Oral Spray as an
Adjuvant Therapy for Mild and Moderate
COVID-19 in Community Health Stations: Clinical
Progression and Viral Clearance Outcomes
DUONG T. TRAN1,2, TRUONG N. PHAM1,3, NHUNG HT NGUYEN4,
HAU D. TRAN5, HUY Q. HOANG6, BO HAN7 and BA X. HOANG7
119-8 Hospital, Ministry of Public Security, Hanoi, Vietnam;
2Faculty of Medicine, Dai Nam University, Hanoi, Vietnam;
3Department of Experimental Surgery and Preclinical Study - University of Medicine and Pharmacy,
Hanoi National University, Hanoi, Vietnam;
4University of Medical Technology, Department of Clinical Laboratory, Hai Duong, Vietnam;
5National Children Hospital, Department of Oncology, Hanoi, Vietnam;
6Natural Health Medical Center, Lawndale, CA, U.S.A.;
7Nimni-Cordoba Tissue Engineering and Drug Discovery Lab, Department of Surgery,
Keck School of Medicine of University of Southern California, Los Angeles, CA, U.S.A.
Abstract. Background/Aim: The aim of this study was to
evaluate the safety and efficacy of AFree oral spray, in
combination with Standard of Care, in treating mild to
moderate COVID-19 patients. This was an open-label,
single-blinded, and controlled randomized clinical trial.
Patients and Methods: The study involved 1,252 patients,
who were randomly assigned to either the control or study
group, with 626 patients in each group. Patients in the
control group were treated with Standard of Care
recommended by the Ministry of Health of Vietnam, while
patients in the study group received AFree oral spray in
addition to Standard of Care for a period of 10 days. The
clinical progression and outcomes of both groups were
compared. Results: The results showed that the proportion
of patients with clinical symptoms on the 5th, 7th and 10th
Correspondence to: Ba X. Hoang, MD, Ph.D., Nimni-Cordoba
Tissue Engineering and Drug Discovery Lab, Department of
Surgery, Keck School of Medicine, University of Southern
California, BMT-302, 1333 San Pablo Street, Los Angeles, CA,
U.S.A. Tel: +1 3234422242, e-mail: baxuanho@usc.edu
Key Words: COVID-19, Zinc, Iodide, dimethyl sulfoxide, DMSO,
AFree, viral infection, SARS-COV-2.
This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY-NC-ND) 4.0
international license (https://creativecommons.org/licenses/by-nc-nd/4.0).
days were significantly lower in the study group (45.05%,
3.19% and 0%, respectively) compared to the control group
(86.10%, 67.73% and 22.84%, respectively). Additionally,
the rate of Real-time PCR test positivity for COVID-19 was
significantly lower in the study group compared to the
control group on the 4th, 7th, and 10th days (82.75% vs.
98.72%, 9.27% vs. 92.97%, and 1.12% vs. 50.48%,
respectively). Furthermore, no side effects or complications
related to AFree oral spray were recorded in the study
group. Conclusion: The use of AFree oral spray resulted in
significant improvements in clinical symptoms, recovery
time, and viral clearance in COVID-19 patients with mild to
moderate symptoms. This therapy has been shown to be safe
and can be used as an adjuvant treatment for COVID-19 as
well as other respiratory viral infections.
COVID-19 is a highly contagious disease that can cause a
wide range of clinical symptoms, ranging from mild to
severe. In the worst cases, it can lead to the development of
respiratory distress syndrome, sepsis, septic shock, and..
DOI record:
{
"DOI": "10.21873/invivo.13313",
"ISSN": [
"0258-851X",
"1791-7549"
],
"URL": "http://dx.doi.org/10.21873/invivo.13313",
"alternative-id": [
"10.21873/invivo.13313"
],
"author": [
{
"affiliation": [],
"family": "TRAN",
"given": "DUONG T.",
"sequence": "first"
},
{
"affiliation": [],
"family": "PHAM",
"given": "TRUONG N.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "NGUYEN",
"given": "NHUNG HT",
"sequence": "additional"
},
{
"affiliation": [],
"family": "TRAN",
"given": "HAU D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "HOANG",
"given": "HUY Q.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "HAN",
"given": "BO",
"sequence": "additional"
},
{
"affiliation": [],
"family": "HOANG",
"given": "BA X.",
"sequence": "additional"
}
],
"container-title": "In Vivo",
"container-title-short": "In Vivo",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
8,
31
]
],
"date-time": "2023-08-31T15:32:07Z",
"timestamp": 1693495927000
},
"deposited": {
"date-parts": [
[
2023,
8,
31
]
],
"date-time": "2023-08-31T15:32:38Z",
"timestamp": 1693495958000
},
"indexed": {
"date-parts": [
[
2023,
9,
1
]
],
"date-time": "2023-09-01T12:12:20Z",
"timestamp": 1693570340761
},
"is-referenced-by-count": 0,
"issue": "5",
"issued": {
"date-parts": [
[
2023
]
]
},
"journal-issue": {
"issue": "5",
"published-online": {
"date-parts": [
[
2023,
8,
31
]
]
},
"published-print": {
"date-parts": [
[
2023
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.21873/invivo.13313",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "9336",
"original-title": [],
"page": "2155-2160",
"prefix": "10.21873",
"published": {
"date-parts": [
[
2023
]
]
},
"published-online": {
"date-parts": [
[
2023,
8,
31
]
]
},
"published-print": {
"date-parts": [
[
2023
]
]
},
"publisher": "Anticancer Research USA Inc.",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "http://iv.iiarjournals.org/lookup/doi/10.21873/invivo.13313"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology",
"General Biochemistry, Genetics and Molecular Biology",
"Cancer Research"
],
"subtitle": [],
"title": "A Prospective Study of AFree Oral Spray as an Adjuvant Therapy for Mild and Moderate COVID-19 in Community Health Stations: Clinical Progression and Viral Clearance Outcomes",
"type": "journal-article",
"volume": "37"
}
