Real-Life Comparison of Mortality in Non-Hospitalised Patients with SARS-CoV-2 Infection at Risk for Clinical Progression Treated with Molnupiravir or Nirmatrevir Plus Ritonavir During the Omicron Era in Italy: A Nationwide, Observational Study
et al., Elsevier BV, doi:10.2139/ssrn.4444431, May 2023
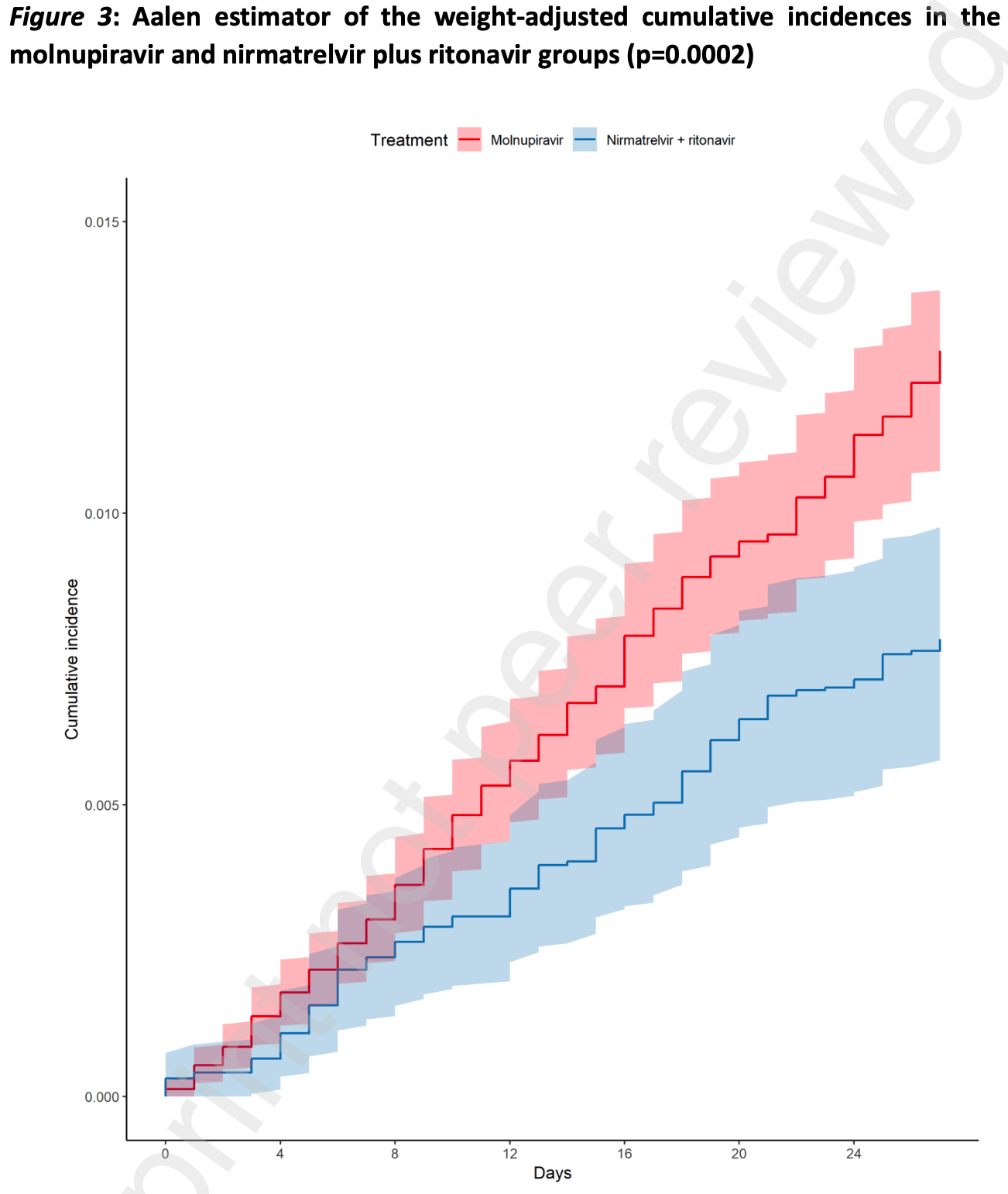
Prospective study of 17,977 outpatients treated with molnupiravir and 11,576 treated with paxlovid, showing significant mortality with both treatments, and lower mortality with paxlovid.
Study covers molnupiravir and paxlovid.
|
risk of death, 32.0% lower, HR 0.68, p < 0.001, treatment 11,576, control 17,977, adjusted per study, Cox proportional hazards.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Torti et al., 10 May 2023, prospective, Italy, preprint, 24 authors, study period 8 February, 2022 - 30 April, 2022, this trial compares with another treatment - results may be better when compared to placebo.
Real-life comparison of mortality in non-hospitalised patients with SARS-CoV-2 infection at risk for clinical progression treated with molnupiravir or nirmatrevir plus ritonavir during the Omicron era in Italy: a nationwide, observational study
Background Comparative data on mortality in COVID-19 patients treated with molnupiravir or with nirmatrelvir plus ritonavir are scarce and inconclusive. In particular, no adequately powered studies have demonstrated statistically significant differences in mortality between the two oral antivirals. We therefore aimed to provide a comparison of all-cause mortality in community-dwelling COVID-19 patients treated during the Omicron era.
Methods In this observational study we used data collected in the nationwide, population-based, cohort of patients registered in the database of the Italian Medicines Agency (AIFA). Patient inclusion in the AIFA registry was mandatory for clinicians to prescribe molnupiravir or nirmatrelvir plus ritonavir. We included in this study patients infected by SARS-CoV-2 treated within 5 days after the test-positive date and symptom onset between February 8 and April 30, 2022. Molnupiravir and nirmatrelvir plus ritonavir all-cause mortality by day 28 was compared after balancing for baseline characteristics using weights obtained from a gradient boosting machine algorithm. Given the nationwide nature of the study, mixed effect Cox regression was performed to account for the underlying variation among Italian regions and National Health System (NHS) centers. Importantly, to increase completeness of the recorded deaths and date correctness, a cross-check with the National Death Registry provided by the Ministry of the Interior was performed.
Findings In the considered timeframe, 31619 patients were registered in the AIFA registry. After exclusion of patients who did not meet the inclusion or the quality control criteria, 17977 patients treated with molnupiravir and 11576 patients with nirmatrelvir plus ritonavir were included in the analysis. Molnupiravir-treated patients were older (median age: 74 years; 47.1% >75 years old) than those treated with nirmatrelvir plus ritonavir (median age: 66.3 years; 29.1% >75 years old). Median time from symptom onset was 3 days in both groups. Mild impairment of renal function, chronic kidney disease, uncontrolled diabetes, cardio-cerebrovascular diseases and asthma requiring daily medications were more frequent in patients who received molnupiravir, while primary or secondary immunodeficiencies and (haemato)-oncological diseases were more frequent in those who received nirmatrelvir plus ritonavir. Most patients received SARS-CoV-2 vaccination (91.8%) with a full vaccine course (86.7%). A higher crude incidence rate of all-cause mortality was found among molnupiravir users (51.83 per 100,000 person-days), compared to nirmatrervir/ritonavir users (22.29 per 100,000 person-days). Comparing the weight-adjusted cumulative incidences using the Aalen estimator, by day 28 the adjusted cumulative incidence rates were 1.23% (95% CI 1.07%-1.38%) for molnupiravir-treated and 0.78% (95% CI 0.58%-0.98%) for nirmatrelvir plus ritonavir-treated patients (adjusted log rank p=0.0002). The..
Conflicts of interest PPO, SC, VS, PR, Giorgio Palù, CT, AS, AV, DT, ET, AP, CB, AG, EME, MF,GBB and ML report no competing interests regarding this article. PB reports research grants and/or personal fees for advisor/consultant and/or speaker/chairman from Viiv, Gilead, Jannsen, Merck and Pfizer. CT reports research grants and/or personal fees for advisor/consultant and/or speaker/chairman from Gilead, Merck, Pfizer, Menarini, GSK, Sanofi, Angelini, thermofischer, Biotest and Diasorin. EN reports research grants and/or personal fees for advisor/consultant and/or speaker/chairman from Gilead, Eli Lilly, Roche, SOBI. BC reports research grants and/or personal fees for advisor/consultant and/or speaker/chairman from Angelini, Menarini. This preprint research paper has not been peer reviewed. Electronic copy available at: https://ssrn.com/abstract=4444431 P r e p r i n t n o t p e e r r e v i e w e d Giustino Parruti reports research grants and/or personal fees for advisor/consultant and/or speaker/chairman from Gilead, Merck, AlphaSigma, Angelini, Pfizer, Lusofarmaco, GSK, Janssen. MB reports research grants and/or personal fees for advisor/consultant and/or speaker/chairman from Angelini, BioMérieux, Cidara, Menarini, MSD, Pfizer and Shionogi. GDP reports research grants and/or personal fees for advisor/consultant and/or speaker/chairman from GS, MSD, ViiV, Abbvie, Janssen, GSK, AZ, Pfizer, Roche. The views expressed in this work are personal and may not be understood or..
References
Bajema, Berry, Streja, Effectiveness of COVID-19 treatment with nirmatrelvir-ritonavir or molnupiravir among U.S. Veterans: target trial emulation studies with one-month and six-month outcome, medRxiv, doi:10.1101/2022.12.05.22283134
Bell, The epigenomic analysis of human obesity, Obesity
Bernal, Da Silva, Musungaie, Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients, N Engl J Med
Bhimraj, Morgan, Shumaker, Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19, Infectious Diseases Society of America
Breccia, Olimpieri, Celant, Management of chronic myeloid leukaemia patients treated with ponatinib in a real-life setting: a retrospective analysis from the monitoring registries of the Italian Medicines Agency (AIFA), Br J Haematol
Buscemi, Davoli, Trecarichi, The three facets of the SARS-CoV-2 pandemic during the first two waves in the northern, central, and southern Italy, J Infect Public Health
Castelnuovo, Bonaccio, Costanzo, Common cardiovascular risk factors and in-hospital mortality in 3,894 patients with COVID-19: survival analysis and machine learning-based findings from the multicentre Italian CORIST Study, Nutr Metab Cardiovasc Dis
Covid-19, COVID-19) Treatment Guidelines. Therapeutic Management of Nonhospitalized Adults With COVID-19
Freund, Schapire, A Decision-Theoretic Generalization of On-Line Learning and an Application to Boosting, J Comput Syst Sci
Gentile, Scotto, Moriello, Nirmatrelvir/Ritonavir and Molnupiravir in the treatment of mild/moderate COVID-19: results of a real-life study, Vaccines
Gisaid, None
Gottlieb, Vaca, Paredes, Early remdesivir to prevent progression to severe Covid-19 in outpatients, N Engl J Med
Hammond, Leister-Tebbe, Gardner, Oral Nirmatrelvir for highrisk, nonhospitalized adults with Covid-19, N Engl J Med
Jdiaa, Mansour, Alayli, COVID-19 and chronic kidney disease: an updated overview of reviews, J Nephrol
Lai, Wang, Chen, The clinical efficacy and safety of antiviral agents for non-hospitalized patients with COVID-19: a systematic review and network meta-analysis of randomized controlled trials, Viruses
Lee, Lessler, Stuart, Improving propensity score weighting using machine learning, Stat Med
Mazzitelli, Mengato, Sasset, Molnupiravir and Nirmatrelvir/Ritonavir: tolerability, safety, and adherence in a retrospective cohort study, Viruses
Mccaffrey, Ridgeway, Morral, Propensity score estimation with boosted regression for evaluating causal effects in observational studies, Psychol Methods
Medicines, Refusal of the marketing authorisation for Lagevrio (molnupiravir)
Medicines, Sospensione di utilizzo del medicinale Lagevrio, molnupiravir
Nirmatrelvir/Ritonavir, Molnupiravir, Remdesivir in a real-world cohort of outpatients with COVID-19 at high risk of progression: the PISA outpatient clinic experience, Infect Dis Ther
Onder, Olimpieri, Celant, Under-prescription of direct oral anticoagulants for treatment of non-valvular atrial fibrillation and venous thromboembolism in the COVID-19 lockdown period, Eur J Prev Cardiol
Paraskevis, Gkova, Mellou, Real-world effectiveness of molnupiravir and nirmatrelvir/ritonavir among COVID-19 community, highly vaccinated patients with high risk for severe disease: Evidence that both antivirals reduce the risk for disease progression and death, medRxiv, doi:10.1101/2023.02.09.23285737
Polverino, Stern, Ruocco, Comorbidities, Cardiovascular Therapies, and COVID-19 Mortality: A Nationwide, Italian Observational Study (ItaliCO), Front Cardiovasc Med
Ripatti, Palmgren, Biometrics, Estimation of multivariate frailty models using penalized partial likelihood, Biometrics
Rosano, Celant, Olimpieri, Impact of the COVID-19 pandemic on prescription of sacubitril/valsartan in Italy, Eur J Heart Fail
Russo, Tacconelli, Olimpieri, Mortality in SARS-CoV-2 hospitalized patients treated with Remdesivir: a nationwide, registrybased study in Italy, Viruses
Tan, Tan, Tan, Long-term kidney function recovery and mortality after COVID-19-associated acute kidney injury: an international multi-centre observational cohort study, eClinicalMedicine
Tiseo, Barbieri, Galfo, Efficacy and safety of
Vulturar, Crivii, Orasan, Obesity Impact on SARS-CoV-2 Infection: Pros and Cons "Obesity Paradox"-A Systematic Review, J Clin Med
Wai, Chan, Cheung, Association of Molnupiravir and Nirmatrelvir-Ritonavir with preventable mortality, hospital admissions and related avoidable healthcare system cost among high-risk patients with mild to moderate COVID-19, Lancet Reg Health West Pac
Who, None
Wok, Tsoi, Isaac, Real-world study on effectiveness of molnupiravir and nirmatrelvir-ritonavir in unvaccinated patients with chronic respiratory diseases with confirmed SARS-CoV-2 infection managed in out-patient setting, Viruses
Wong, Au, Lau, Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study, Lancet
Xie, Liu, Adjusted Kaplan-Meier estimator and log-rank test with inverse probability of treatment weighting for survival data, Stat Med
Yang, Lorenzi, Papadogeorgou, Propensity score weighting for causal subgroup analysis, Stat Med
Zhang, Kim, Lonjon, Balance diagnostics after propensity score matching, Ann Transl Med
DOI record:
{
"DOI": "10.2139/ssrn.4444431",
"URL": "http://dx.doi.org/10.2139/ssrn.4444431",
"author": [
{
"affiliation": [],
"family": "Torti",
"given": "Carlo",
"sequence": "first"
},
{
"affiliation": [],
"family": "Olimpieri",
"given": "Pier Paolo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bonfanti",
"given": "Paolo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tascini",
"given": "Carlo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Celant",
"given": "Simone",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tacconi",
"given": "Danilo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nicastri",
"given": "Emanuele",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tacconelli",
"given": "Evelina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cacopardo",
"given": "Bruno",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Perrella",
"given": "Alessandro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Buccoliero",
"given": "Giovanni Battista",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Parruti",
"given": "Giustino",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bassetti",
"given": "Matteo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Biagetti",
"given": "Carlo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Giacometti",
"given": "Andrea",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Erne",
"given": "Elke Maria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Frontuto",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lanzafame",
"given": "Massimiliano",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Valentina",
"given": "Summa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Spagnoli",
"given": "Alessandra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vestri",
"given": "Annarita",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Di Perri",
"given": "Giovanni",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Russo",
"given": "Pierluigi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Palù",
"given": "Giorgio",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
5,
11
]
],
"date-time": "2023-05-11T03:43:00Z",
"timestamp": 1683776580000
},
"deposited": {
"date-parts": [
[
2023,
5,
11
]
],
"date-time": "2023-05-11T03:43:00Z",
"timestamp": 1683776580000
},
"group-title": "SSRN",
"indexed": {
"date-parts": [
[
2023,
5,
12
]
],
"date-time": "2023-05-12T04:41:01Z",
"timestamp": 1683866461026
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023
]
]
},
"member": "78",
"original-title": [],
"posted": {
"date-parts": [
[
2023
]
]
},
"prefix": "10.2139",
"published": {
"date-parts": [
[
2023
]
]
},
"publisher": "Elsevier BV",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.ssrn.com/abstract=4444431"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "Real-Life Comparison of Mortality in Non-Hospitalised Patients with SARS-CoV-2 Infection at Risk for Clinical Progression Treated with Molnupiravir or Nirmatrevir Plus Ritonavir During the Omicron Era in Italy: A Nationwide, Observational Study",
"type": "posted-content"
}