Adherence to application technique of inhaled corticosteroid in patients with asthma and COVID-19 improves outcomes
et al., BMJ Open Respiratory Research, doi:10.1136/bmjresp-2023-001874, Jan 2024
Budesonide for COVID-19
27th treatment shown to reduce risk in
September 2021, now with p = 0.0000042 from 14 studies, recognized in 10 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
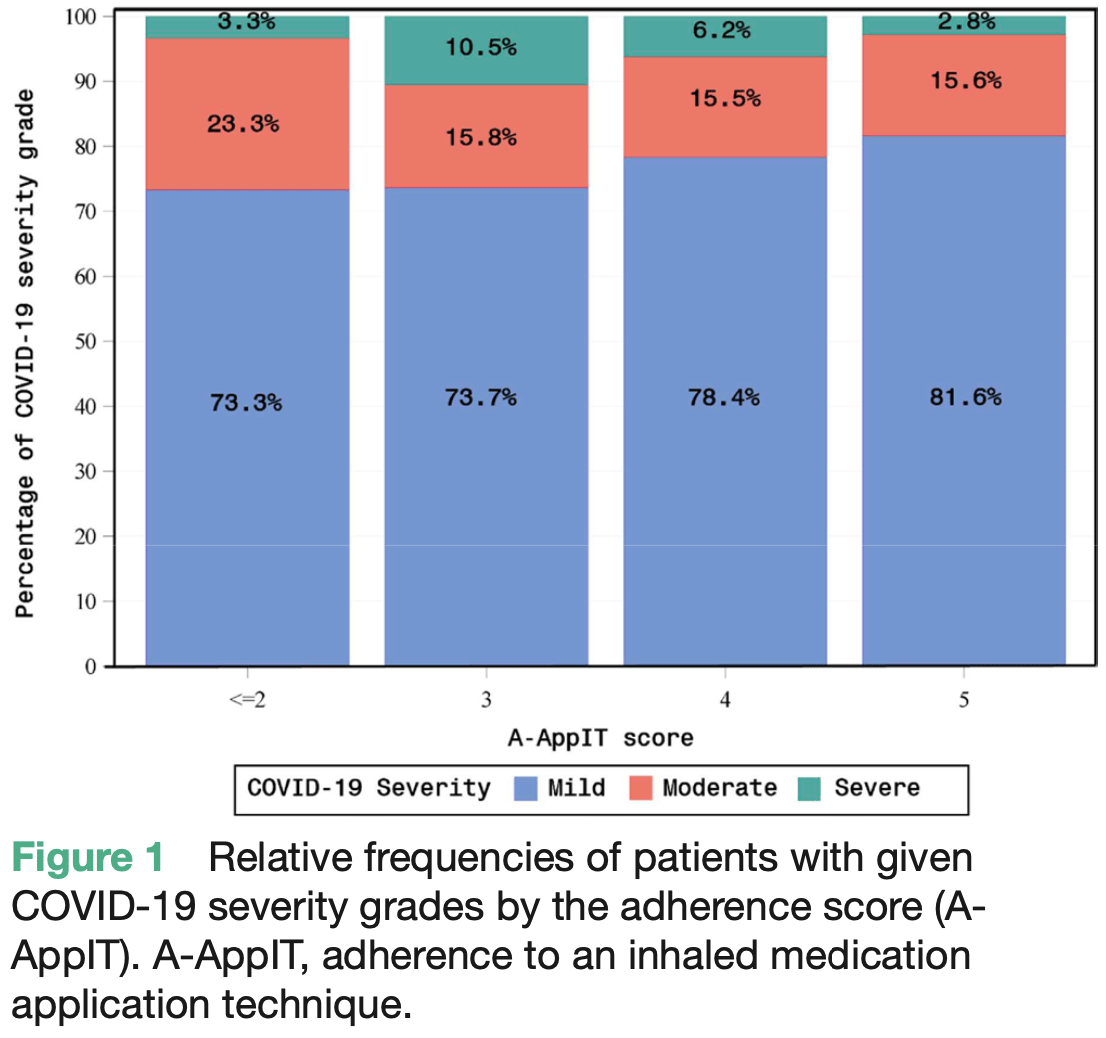
Retrospective 654 asthma patients in the Czech Republic and Slovakia diagnosed with COVID-19 showing improved COVID-19 outcomes, including lower risk of hospitalization and faster recovery of lung function, with better adherence to inhaled corticosteroid (ICS) technique measured by a 5-step assessment score. Scoring higher on the assessment was associated with significantly lower COVID-19 severity after adjusting for age and asthma severity. The assessment score was also associated with higher quality of life scores after COVID-19. The results highlight the importance of proper ICS inhaler technique and adherence monitoring to optimize COVID-19 outcomes.
Tichopád et al., 6 Jan 2024, retrospective, multiple countries, peer-reviewed, mean age 61.8, 8 authors.
Contact: vratislav.sedlak@fnhk.cz.
Adherence to application technique of inhaled corticosteroid in patients with asthma and COVID-19 improves outcomes
BMJ Open Respiratory Research, doi:10.1136/bmjresp-2023-001874
Background Inhaled corticosteroids have been widely reported as a preventive measure against the development of severe forms of COVID-19 not only in patients with asthma. Methods In 654 Czech and Slovak patients with asthma who developed COVID-19, we investigated whether the correct use of inhaler containing corticosteroids was associated with a less severe course of COVID-19 and whether this had an impact on the need for hospitalisation, measurable lung functions and quality of life (QoL). Results Of the studied cohort 51.4% had moderate persistent, 29.9% mild persistent and 7.2% severe persistent asthma. We found a significant adverse effect of poor inhaler adherence on COVID-19 severity (p=0.049). We also observed a lower hospitalisation rate in patients adequately taking the inhaler with OR of 0.83. Vital capacity and forced expiratory lung volume deterioration caused by COVID-19 were significantly reversed, by approximately twofold to threefold, in individuals who inhaled correctly.
Conclusion Higher quality of inhalation technique of corticosteroids measured by adherence to an inhaled medication application technique (A-AppIT ) score had a significant positive effect on reversal of the vital capacity and forced expiratory lung volume in 1 s worsening (p=0.027 and p<0.0001, respectively) due to COVID-19. Scoring higher in the A-AppIT was also associated with significantly improved QoL. All measured variables concordantly and without exception showed a positive improvement in response to better adherence. We suggest that corticosteroids provide protection against the worsening of lungs in patients with COVID-19 and that correct and easily assessable adherence to corticosteroids with appropriate inhalation technique play an important role in preventing severe form of COVID-19. ⇒ As the importance of addressing the needs of the ageing population worldwide continues to grow, potential declines in adherence to inhalation techniques due to factors such as diminished motor skills and cognitive function may present additional challenges. Improper inhaler usage can persist unnoticed in patients over extended periods, eluding physician detection. Hence, the necessity of implementing a straightforward and all-encompassing assessment protocol during regular clinical visits becomes paramount.
References
Bartoletti, Azap, Barac, ESCMID COVID-19 living guidelines: drug treatment and clinical management, Clin Microbiol Infect, doi:10.1016/j.cmi.2021.11.007
Bloom, Drake, Docherty, Risk of adverse outcomes in patients with underlying respiratory conditions admitted to hospital with COVID-19: a national, multicentre prospective cohort study using the ISARIC WHO clinical characterisation protocol UK, Lancet Respir Med, doi:10.1016/S2213-2600(21)00013-8
Capstick, Clifton, Inhaler technique and training in people with chronic obstructive pulmonary disease and asthma, Expert Rev Respir Med, doi:10.1586/ers.11.89
Cloutier, Dixon, Krishnan, Managing asthma in adolescents and adults: 2020 asthma guideline update from the National Asthma Education and Prevention Program, JAMA, doi:10.1001/jama.2020.21974
Devlin, Shah, Feng, Valuing health-related quality of life: an EQ-5D-5L value set for England, Health Econ, doi:10.1002/hec.3564
Grasselli, Zangrillo, Zanella, Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to Icus of the Lombardy region, JAMA, doi:10.1001/jama.2020.5394
Griesel, Wagner, Mikolajewska, Inhaled corticosteroids for the treatment of COVID-19, Cochrane Database Syst Rev, doi:10.1002/14651858.CD015125
Halpin, Faner, Sibila, Do chronic respiratory diseases or their treatment affect the risk of SARS-CoV-2 infection, Lancet Respir Med, doi:10.1016/S2213-2600(20)30167-3
Halpin, Singh, Hadfield, Inhaled corticosteroids and COVID-19: a systematic review and clinical perspective, Eur Respir J, doi:10.1183/13993003.01009-2020
Johnston, Asthma and COVID-19: is asthma a risk factor for severe outcomes, Allergy, doi:10.1111/all.14348
Kwah, Peters, Asthma in adults: principles of treatment, Allergy Asthma Proc, doi:10.2500/aap.2019.40.4256
Laube, Janssens, De Jongh, What the pulmonary specialist should know about the new inhalation therapies, Eur Respir J, doi:10.1183/09031936.00166410
Li, Yan, Gao, Effectiveness of corticosteroids to treat severe COVID-19: a systematic review and meta-analysis of prospective studies, Int Immunopharmacol, doi:10.1016/j.intimp.2021.108121
Lipworth, Chan, Kuo, inhaled corticosteroids in asthma and Coronavirus disease 2019: keep calm and carry on, Ann Allergy Asthma Immunol, doi:10.1016/j.anai.2020.06.026
Luczak-Wozniak, Dabrowska, Domagala, Mishandling of pMDI and DPI Inhalers in asthma and COPD -repetitive and nonrepetitive errors, Pulm Pharmacol Ther, doi:10.1016/j.pupt.2018.06.002
Matsuyama, Kawase, Nao, The inhaled steroid ciclesonide blocks SARS-CoV-2 RNA replication by targeting the viral replication-transcription complex in cultured cells, J Virol, doi:10.1128/JVI.01648-20
Merad, Blish, Sallusto, The immunology and immunopathology of COVID-19, Science, doi:10.1126/science.abm8108
Myles, Nguyen-Van-Tam, Semple, Differences between asthmatics and nonasthmatics hospitalised with influenza a infection, Eur Respir J, doi:10.1183/09031936.00015512
Nice, Inhaled corticosteroid doses for NICE's asthma guideline
Panettieri, Carson, Horton, Asthma and COVID: what are the important questions?, J Allergy Clin Immunol Pract, doi:10.1016/j.jaip.2020.06.008
Peters, Fahy, COVID-19-related genes in sputum cells in asthma. Relationship to demographic features and corticosteroids, Am J Respir Crit Care Med, doi:10.1164/rccm.v202erratum7
Plaza, Giner, Rodrigo, Errors in the use of inhalers by health care professionals: a systematic review, J Allergy Clin Immunol Pract, doi:10.1016/j.jaip.2017.12.032
Poudel, Zhu, Cooper, Impact of covid-19 on healthrelated quality of life of patients: a structured review, PLoS ONE, doi:10.1371/journal.pone.0259164
Ramakrishnan, Nicolau, Langford, Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial, Lancet Respir Med, doi:10.1016/S2213-2600(21)00160-0
Reddel, Bacharier, Bateman, Global initiative for asthma strategy 2021 executive summary and rationale for key changes, Am J Respir Crit Care Med, doi:10.1164/rccm.202109-2205PP
Richardson, Hirsch, Narasimhan, Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area, JAMA, doi:10.1001/jama.2020.6775
Rogliani, Ora, Puxeddu, Adherence to COPD treatment: myth and reality, Respir Med, doi:10.1016/j.rmed.2017.06.007
Sahu, Mathew, Bhat, Steroids use in non-oxygen requiring COVID-19 patients: a systematic review and meta-analysis, QJM, doi:10.1093/qjmed/hcab212
Suda, Tsuruta, Eom, Acute lung injury induces cardiovascular dysfunction: effects of IL-6 and budesonide/ formoterol, Am J Respir Cell Mol Biol, doi:10.1165/rcmb.2010-0169OC
Usmani, Lavorini, Marshall, Critical inhaler errors in asthma and COPD: a systematic review of impact on health outcomes, Respir Res, doi:10.1186/s12931-017-0710-y
Venkatesan, GINA report for asthma, Lancet Respir Med, doi:10.1016/S2213-2600(23)00230-8
Vytrisalova, Hendrychova, Touskova, Breathing out completely before inhalation: the most problematic step in application technique in patients with non-mild chronic obstructive pulmonary disease, Front Pharmacol, doi:10.3389/fphar.2019.00241
Yamaya, Nishimura, Deng, Inhibitory effects of glycopyrronium, formoterol, and budesonide on Coronavirus Hcov-229E replication and cytokine production by primary cultures of human nasal and tracheal epithelial cells, Respir Investig, doi:10.1016/j.resinv.2019.12.005
Yu, Bafadhel, Dorward, Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial, Lancet, doi:10.1016/S0140-6736(21)01744-X
DOI record:
{
"DOI": "10.1136/bmjresp-2023-001874",
"ISSN": [
"2052-4439"
],
"URL": "http://dx.doi.org/10.1136/bmjresp-2023-001874",
"abstract": "<jats:sec><jats:title>Background</jats:title><jats:p>Inhaled corticosteroids have been widely reported as a preventive measure against the development of severe forms of COVID-19 not only in patients with asthma.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>In 654 Czech and Slovak patients with asthma who developed COVID-19, we investigated whether the correct use of inhaler containing corticosteroids was associated with a less severe course of COVID-19 and whether this had an impact on the need for hospitalisation, measurable lung functions and quality of life (QoL).</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>Of the studied cohort 51.4% had moderate persistent, 29.9% mild persistent and 7.2% severe persistent asthma. We found a significant adverse effect of poor inhaler adherence on COVID-19 severity (p=0.049). We also observed a lower hospitalisation rate in patients adequately taking the inhaler with OR of 0.83. Vital capacity and forced expiratory lung volume deterioration caused by COVID-19 were significantly reversed, by approximately twofold to threefold, in individuals who inhaled correctly.</jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p>Higher quality of inhalation technique of corticosteroids measured by adherence to an inhaled medication application technique (A-AppIT) score had a significant positive effect on reversal of the vital capacity and forced expiratory lung volume in 1 s worsening (p=0.027 and p<0.0001, respectively) due to COVID-19. Scoring higher in the A-AppIT was also associated with significantly improved QoL. All measured variables concordantly and without exception showed a positive improvement in response to better adherence. We suggest that corticosteroids provide protection against the worsening of lungs in patients with COVID-19 and that correct and easily assessable adherence to corticosteroids with appropriate inhalation technique play an important role in preventing severe form of COVID-19.</jats:p></jats:sec>",
"alternative-id": [
"10.1136/bmjresp-2023-001874"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-1308-0877",
"affiliation": [],
"authenticated-orcid": false,
"family": "Tichopád",
"given": "Aleš",
"sequence": "first"
},
{
"affiliation": [],
"family": "Žigmond",
"given": "Jan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jeseňák",
"given": "Miloš",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Solovič",
"given": "Ivan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Breciková",
"given": "Katarína",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rybář",
"given": "Marian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rožánek",
"given": "Martin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sedlák",
"given": "Vratislav",
"sequence": "additional"
}
],
"container-title": "BMJ Open Respiratory Research",
"container-title-short": "BMJ Open Resp Res",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"bmj.com"
]
},
"created": {
"date-parts": [
[
2024,
1,
10
]
],
"date-time": "2024-01-10T05:50:55Z",
"timestamp": 1704865855000
},
"deposited": {
"date-parts": [
[
2024,
1,
10
]
],
"date-time": "2024-01-10T05:51:09Z",
"timestamp": 1704865869000
},
"funder": [
{
"DOI": "10.13039/100007655",
"award": [
"SGS23/197/OHK5/3T/17"
],
"doi-asserted-by": "publisher",
"name": "České Vysoké Učení Technické v Praze"
},
{
"DOI": "10.13039/100007560",
"doi-asserted-by": "publisher",
"name": "Chiesi Farmaceutici"
},
{
"DOI": "10.13039/100007397",
"award": [
"00179906"
],
"doi-asserted-by": "publisher",
"name": "Univerzita Karlova v Praze"
},
{
"DOI": "10.13039/100019624",
"award": [
"00179906"
],
"doi-asserted-by": "publisher",
"name": "Fakultní nemocnice Hradec Králové"
}
],
"indexed": {
"date-parts": [
[
2024,
1,
11
]
],
"date-time": "2024-01-11T00:25:35Z",
"timestamp": 1704932735211
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2024,
1
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2024,
1,
6
]
]
},
"published-print": {
"date-parts": [
[
2024,
1
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "unspecified",
"delay-in-days": 5,
"start": {
"date-parts": [
[
2024,
1,
6
]
],
"date-time": "2024-01-06T00:00:00Z",
"timestamp": 1704499200000
}
}
],
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1136/bmjresp-2023-001874",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "239",
"original-title": [],
"page": "e001874",
"prefix": "10.1136",
"published": {
"date-parts": [
[
2024,
1
]
]
},
"published-online": {
"date-parts": [
[
2024,
1,
6
]
]
},
"published-print": {
"date-parts": [
[
2024,
1
]
]
},
"publisher": "BMJ",
"reference": [
{
"DOI": "10.1016/S0140-6736(22)00172-6",
"article-title": "Pandemic preparedness and COVID-19: an exploratory analysis of infection and fatality rates, and contextual factors associated with preparedness in 177 countries, from Jan 1, 2020, to Sept 30, 2021",
"doi-asserted-by": "crossref",
"first-page": "1489",
"journal-title": "Lancet",
"key": "2024010921481660000_11.1.e001874.1",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.1126/science.abm8108",
"doi-asserted-by": "publisher",
"key": "2024010921481660000_11.1.e001874.2"
},
{
"DOI": "10.1016/j.cmi.2021.11.007",
"article-title": "ESCMID COVID-19 living guidelines: drug treatment and clinical management",
"author": "Bartoletti",
"doi-asserted-by": "crossref",
"first-page": "222",
"journal-title": "Clin Microbiol Infect",
"key": "2024010921481660000_11.1.e001874.3",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1093/qjmed/hcab212",
"article-title": "Steroids use in non-oxygen requiring COVID-19 patients: a systematic review and meta-analysis",
"author": "Sahu",
"doi-asserted-by": "crossref",
"first-page": "455",
"journal-title": "QJM",
"key": "2024010921481660000_11.1.e001874.4",
"volume": "114",
"year": "2021"
},
{
"DOI": "10.1164/rccm.202109-2205PP",
"doi-asserted-by": "publisher",
"key": "2024010921481660000_11.1.e001874.5"
},
{
"DOI": "10.1001/jama.2020.21974",
"article-title": "Managing asthma in adolescents and adults: 2020 asthma guideline update from the National Asthma Education and Prevention Program",
"author": "Cloutier",
"doi-asserted-by": "crossref",
"first-page": "2301",
"journal-title": "JAMA",
"key": "2024010921481660000_11.1.e001874.6",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.2500/aap.2019.40.4256",
"article-title": "Asthma in adults: principles of treatment",
"author": "Kwah",
"doi-asserted-by": "crossref",
"first-page": "396",
"journal-title": "Allergy Asthma Proc",
"key": "2024010921481660000_11.1.e001874.7",
"volume": "40",
"year": "2019"
},
{
"DOI": "10.1016/S2213-2600(23)00230-8",
"article-title": "GINA report for asthma",
"author": "Venkatesan",
"doi-asserted-by": "crossref",
"first-page": "589",
"journal-title": "Lancet Respir Med",
"key": "2024010921481660000_11.1.e001874.8",
"volume": "11",
"year": "2023"
},
{
"DOI": "10.1111/all.14348",
"article-title": "Asthma and COVID-19: is asthma a risk factor for severe outcomes",
"author": "Johnston",
"doi-asserted-by": "crossref",
"first-page": "1543",
"journal-title": "Allergy",
"key": "2024010921481660000_11.1.e001874.9",
"volume": "75",
"year": "2020"
},
{
"DOI": "10.1016/S2213-2600(21)00013-8",
"article-title": "Risk of adverse outcomes in patients with underlying respiratory conditions admitted to hospital with COVID-19: a national, multicentre prospective cohort study using the ISARIC WHO clinical characterisation protocol UK",
"author": "Bloom",
"doi-asserted-by": "crossref",
"first-page": "699",
"journal-title": "Lancet Respir Med",
"key": "2024010921481660000_11.1.e001874.10",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1016/j.anai.2020.06.026",
"article-title": "Use of inhaled corticosteroids in asthma and Coronavirus disease 2019: keep calm and carry on",
"author": "Lipworth",
"doi-asserted-by": "crossref",
"first-page": "503",
"journal-title": "Ann Allergy Asthma Immunol",
"key": "2024010921481660000_11.1.e001874.11",
"volume": "125",
"year": "2020"
},
{
"DOI": "10.1165/rcmb.2010-0169OC",
"doi-asserted-by": "publisher",
"key": "2024010921481660000_11.1.e001874.12"
},
{
"DOI": "10.1016/j.resinv.2019.12.005",
"doi-asserted-by": "publisher",
"key": "2024010921481660000_11.1.e001874.13"
},
{
"DOI": "10.1183/13993003.01009-2020",
"doi-asserted-by": "crossref",
"key": "2024010921481660000_11.1.e001874.14",
"unstructured": "Halpin DMG , Singh D , Hadfield RM . Inhaled corticosteroids and COVID-19: a systematic review and clinical perspective. Eur Respir J 2020;55:2001009. doi:10.1183/13993003.01009-2020"
},
{
"DOI": "10.1016/S2213-2600(21)00160-0",
"article-title": "Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial",
"author": "Ramakrishnan",
"doi-asserted-by": "crossref",
"first-page": "763",
"journal-title": "Lancet Respir Med",
"key": "2024010921481660000_11.1.e001874.15",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1183/09031936.00166410",
"doi-asserted-by": "publisher",
"key": "2024010921481660000_11.1.e001874.16"
},
{
"DOI": "10.1016/j.rmed.2017.06.007",
"article-title": "Adherence to COPD treatment: myth and reality",
"author": "Rogliani",
"doi-asserted-by": "crossref",
"first-page": "117",
"journal-title": "Respir Med",
"key": "2024010921481660000_11.1.e001874.17",
"volume": "129",
"year": "2017"
},
{
"DOI": "10.3389/fphar.2019.00241",
"doi-asserted-by": "crossref",
"key": "2024010921481660000_11.1.e001874.18",
"unstructured": "Vytrisalova M , Hendrychova T , Touskova T , et al . Breathing out completely before inhalation: the most problematic step in application technique in patients with non-mild chronic obstructive pulmonary disease. Front Pharmacol 2019;10:241. doi:10.3389/fphar.2019.00241"
},
{
"DOI": "10.1016/j.jaip.2017.12.032",
"article-title": "Errors in the use of inhalers by health care professionals: a systematic review",
"author": "Plaza",
"doi-asserted-by": "crossref",
"first-page": "987",
"journal-title": "J Allergy Clin Immunol Pract",
"key": "2024010921481660000_11.1.e001874.19",
"volume": "6",
"year": "2018"
},
{
"DOI": "10.1586/ers.11.89",
"article-title": "Inhaler technique and training in people with chronic obstructive pulmonary disease and asthma",
"author": "Capstick",
"doi-asserted-by": "crossref",
"first-page": "91",
"journal-title": "Expert Rev Respir Med",
"key": "2024010921481660000_11.1.e001874.20",
"volume": "6",
"year": "2012"
},
{
"DOI": "10.1016/j.pupt.2018.06.002",
"article-title": "Mishandling of pMDI and DPI Inhalers in asthma and COPD - repetitive and non-repetitive errors",
"author": "Luczak-Wozniak",
"doi-asserted-by": "crossref",
"first-page": "65",
"journal-title": "Pulm Pharmacol Ther",
"key": "2024010921481660000_11.1.e001874.21",
"volume": "51",
"year": "2018"
},
{
"DOI": "10.1186/s12931-017-0710-y",
"doi-asserted-by": "crossref",
"key": "2024010921481660000_11.1.e001874.22",
"unstructured": "Usmani OS , Lavorini F , Marshall J , et al . Critical inhaler errors in asthma and COPD: a systematic review of impact on health outcomes. Respir Res 2018;19:10. doi:10.1186/s12931-017-0710-y"
},
{
"key": "2024010921481660000_11.1.e001874.23",
"unstructured": "NICE . Inhaled corticosteroid doses for NICE’s asthma guideline. 2018."
},
{
"DOI": "10.1371/journal.pone.0259164",
"doi-asserted-by": "crossref",
"key": "2024010921481660000_11.1.e001874.24",
"unstructured": "Poudel AN , Zhu S , Cooper N , et al . Impact of covid-19 on health-related quality of life of patients: a structured review. PLoS ONE 2021;16:e0259164. doi:10.1371/journal.pone.0259164"
},
{
"DOI": "10.1002/hec.3564",
"doi-asserted-by": "publisher",
"key": "2024010921481660000_11.1.e001874.25"
},
{
"DOI": "10.1016/S0140-6736(21)01744-X",
"doi-asserted-by": "publisher",
"key": "2024010921481660000_11.1.e001874.26"
},
{
"DOI": "10.1002/14651858.CD015125",
"doi-asserted-by": "crossref",
"key": "2024010921481660000_11.1.e001874.27",
"unstructured": "Griesel M , Wagner C , Mikolajewska A , et al . Inhaled corticosteroids for the treatment of COVID-19. Cochrane Database Syst Rev 2022;3:CD015125. doi:10.1002/14651858.CD015125"
},
{
"DOI": "10.1128/JVI.01648-20",
"doi-asserted-by": "crossref",
"key": "2024010921481660000_11.1.e001874.28",
"unstructured": "Matsuyama S , Kawase M , Nao N , et al . The inhaled steroid ciclesonide blocks SARS-CoV-2 RNA replication by targeting the viral replication-transcription complex in cultured cells. J Virol 2020;95:e01648-20. doi:10.1128/JVI.01648-20"
},
{
"DOI": "10.1016/j.intimp.2021.108121",
"article-title": "Effectiveness of corticosteroids to treat severe COVID-19: a systematic review and meta-analysis of prospective studies",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "108121",
"journal-title": "Int Immunopharmacol",
"key": "2024010921481660000_11.1.e001874.29",
"volume": "100",
"year": "2021"
},
{
"DOI": "10.1183/09031936.00015512",
"doi-asserted-by": "publisher",
"key": "2024010921481660000_11.1.e001874.30"
},
{
"DOI": "10.1001/jama.2020.5394",
"article-title": "Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to Icus of the Lombardy region",
"author": "Grasselli",
"doi-asserted-by": "crossref",
"first-page": "1574",
"journal-title": "JAMA",
"key": "2024010921481660000_11.1.e001874.31",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.6775",
"doi-asserted-by": "publisher",
"key": "2024010921481660000_11.1.e001874.32"
},
{
"DOI": "10.1016/S2213-2600(20)30167-3",
"article-title": "Do chronic respiratory diseases or their treatment affect the risk of SARS-CoV-2 infection",
"author": "Halpin",
"doi-asserted-by": "crossref",
"first-page": "436",
"journal-title": "Lancet Respir Med",
"key": "2024010921481660000_11.1.e001874.33",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1016/j.jaip.2020.06.008",
"article-title": "Asthma and COVID: what are the important questions?",
"author": "Panettieri",
"doi-asserted-by": "crossref",
"first-page": "2487",
"journal-title": "J Allergy Clin Immunol Pract",
"key": "2024010921481660000_11.1.e001874.34",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1164/rccm.v202erratum7",
"article-title": "COVID-19–related genes in sputum cells in asthma. Relationship to demographic features and corticosteroids",
"author": "Peters",
"doi-asserted-by": "crossref",
"first-page": "1744",
"journal-title": "Am J Respir Crit Care Med",
"key": "2024010921481660000_11.1.e001874.35",
"volume": "202",
"year": "2020"
}
],
"reference-count": 35,
"references-count": 35,
"relation": {},
"resource": {
"primary": {
"URL": "https://bmjopenrespres.bmj.com/lookup/doi/10.1136/bmjresp-2023-001874"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pulmonary and Respiratory Medicine"
],
"subtitle": [],
"title": "Adherence to application technique of inhaled corticosteroid in patients with asthma and COVID-19 improves outcomes",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1136/crossmarkpolicy",
"volume": "11"
}
