A randomized placebo-controlled trial of convalescent plasma for adults hospitalized with COVID-19 pneumonia
et al., Scientific Reports, doi:10.1038/s41598-022-19629-z, CCAP-2, NCT04345289, Sep 2022
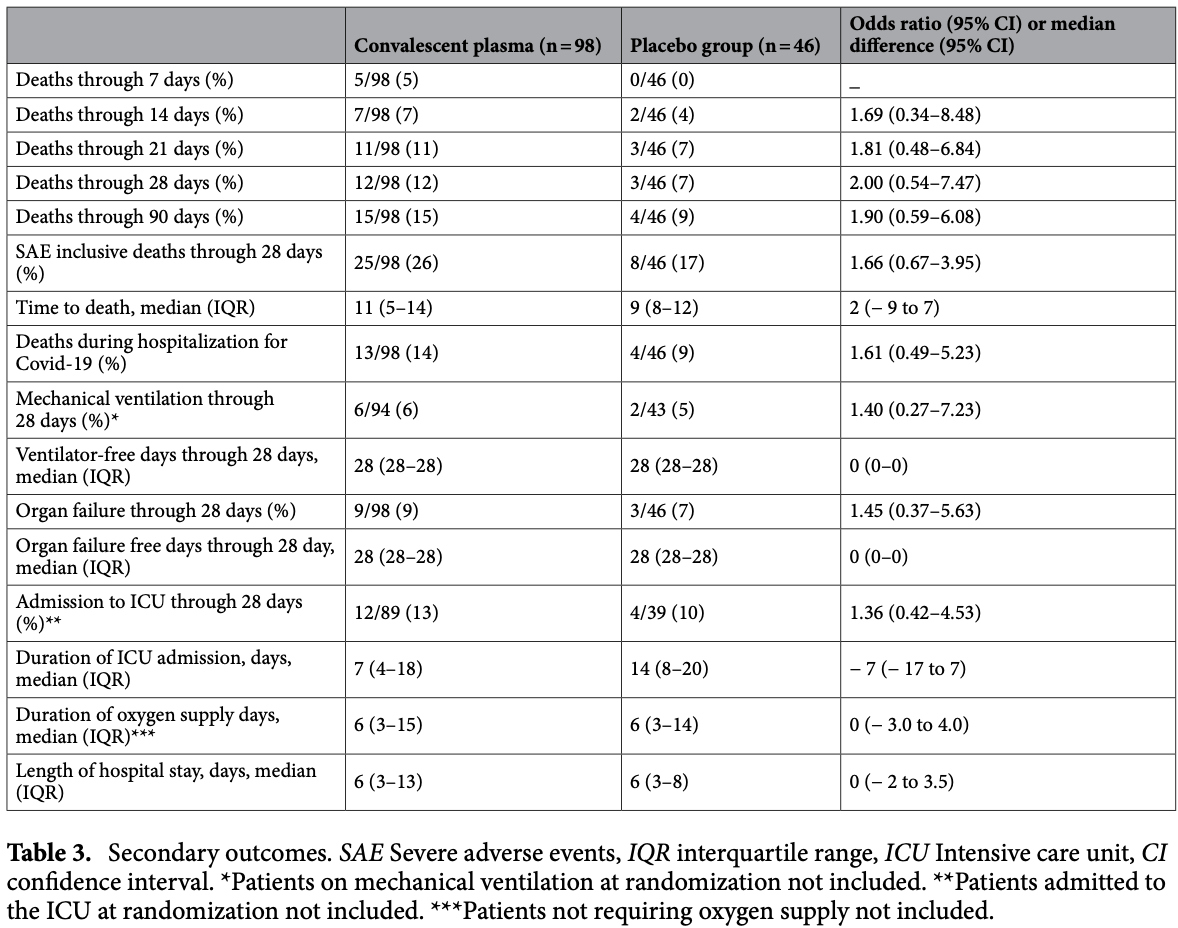
RCT 147 patients in Denmark, showing no significant difference in outcomes with convalescent plasma. The trial was terminated due to futility.
|
risk of death, 76.0% higher, RR 1.76, p = 0.43, treatment 15 of 98 (15.3%), control 4 of 46 (8.7%), day 90.
|
|
risk of death, 87.8% higher, RR 1.88, p = 0.39, treatment 12 of 98 (12.2%), control 3 of 46 (6.5%), day 28.
|
|
risk of death, 72.1% higher, RR 1.72, p = 0.55, treatment 11 of 98 (11.2%), control 3 of 46 (6.5%), day 21.
|
|
risk of death, 64.3% higher, RR 1.64, p = 0.72, treatment 7 of 98 (7.1%), control 2 of 46 (4.3%), day 14.
|
|
risk of death, 734.7% higher, RR 8.35, p = 0.18, treatment 5 of 98 (5.1%), control 0 of 46 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm), day 7.
|
|
risk of mechanical ventilation, 37.2% higher, RR 1.37, p = 1.00, treatment 6 of 94 (6.4%), control 2 of 43 (4.7%), day 28.
|
|
risk of ICU admission, 31.5% higher, RR 1.31, p = 0.77, treatment 12 of 89 (13.5%), control 4 of 39 (10.3%), day 28.
|
|
risk of 7-point scale, 41.0% higher, OR 1.41, p = 0.32, treatment 98, control 46, day 14, primary outcome, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Thorlacius-Ussing et al., 30 Sep 2022, Double Blind Randomized Controlled Trial, placebo-controlled, Denmark, peer-reviewed, 27 authors, study period 13 June, 2020 - 16 March, 2021, average treatment delay 11.0 days, trial NCT04345289 (history) (CCAP-2).
Contact: louise.thorlacius-ussing@regionh.dk.
A randomized placebo-controlled trial of convalescent plasma for adults hospitalized with COVID-19 pneumonia
Scientific Reports, doi:10.1038/s41598-022-19629-z
Passive immunotherapy with convalescent plasma may be the only available agent during the early phases of a pandemic. Here, we report safety and efficacy of high-titer convalescent plasma for COVID-19 pneumonia. Double-blinded randomized multicenter placebo-controlled trial of adult patients hospitalized with COVID-19 pneumonia. The intervention was COVID-19 convalescent plasma and placebo was saline allocated 2:1. The primary outcome was clinical status 14 days after the intervention evaluated on a clinical ordinal scale. The trial was registered at ClinicalTrials.Gov, NCT04345289, 14/04/2020. The CCAP-2 trial was terminated prematurely due to futility. Of 147 patients randomized, we included 144 patients in the modified intention-to-treat population. The ordinal clinical status 14 days post-intervention was comparable between treatment groups (odds ratio (OR) 1.41, 95% confidence interval (CI) 0.72-2.09). Results were consistent when evaluating clinical progression on an individual level 14 days after intervention (OR 1.09; 95% CI 0.46-1.73). No significant differences in length of hospital stay, admission to ICU, frequency of severe adverse events or all-cause mortality during follow-up were found between the intervention and the placebo group. Infusion of convalescent plasma did not influence clinical progression, survival or length of hospitalization in patients with COVID-19 pneumonia. Early in a pandemic, such as the ongoing outbreak of the coronavirus disease-19 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), it is unlikely that specific therapeutic agents will be available. Plasma collected from a patient convalescent from SARS-CoV-2 infection contains neutralizing antibodies against SARS-CoV-2 with a possible therapeutic role for passive immunotherapy. Passive immunotherapy
Author contributions
Competing interests Dr Nielsen declares grants from Novo Nordisk Foundation and personal fees from GSK and MDS outside of the submitted work. Dr Østergaard reports personal fees from Sanofi. Dr Erikstrup reports grants from Abbots diagnostic outside of the submitted work. Dr Benfield reports grants from Novo Nordisk Foundation, grants from Simonsen Foundation, grants and personal fees from GSK, grants and personal fees from Pfizer, personal fees from Boehringer Ingelheim, personal fees from Abbvie, grants and personal fees from Gilead, personal fees from MSD, grants from Lundbeck Foundation, grants from Kai Hansen Foundation, and grants from Erik and Susanna Olsen's Charitable Fund outside the submitted work. All other authors have nothing to disclose.
Additional information
Supplementary Information The online version contains supplementary material available at https:// doi. org/ 10. 1038/ s41598-022-19629-z. Correspondence and requests for materials should be addressed to L.T.-U. Reprints and permissions information is available at www.nature.com/reprints. Publisher's note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Agarwal, Convalescent plasma in the management of moderate covid-19 in adults in India: Open label phase II multicentre randomised controlled trial (PLACID Trial), BMJ
Avendaño-Solá, A multicenter randomized open-label clinical trial for convalescent plasma in patients hospitalized with COVID-19 pneumonia, J Clin Invest
Beigel, Remdesivir for the treatment of Covid-19: Final report, N. Engl. J. Med
Georgsen, Bagge Hansen, Sørensen, Standarder, None
Harris, Research electronic data capture (REDCap): A metadata-driven methodology and workflow process for providing translational research informatics support, J. Biomed. Inform
Harris, The REDCap consortium: Building an international community of software platform partners, J. Biomed. Inform
Harritshøj, Comparison of 16 serological SARS-CoV-2 immunoassays in 16 clinical laboratories, J. Clin. Microbiol
Hung, Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection, Clin. Infect. Dis
Janiaud, Association of convalescent plasma treatment with clinical outcomes in patients with COVID-19: A systematic review and meta-analysis, JAMA
Joyner, Convalescent plasma antibody levels and the risk of death from Covid-19, N. Engl. J. Med
Korley, Early convalescent plasma for high-risk outpatients with Covid-19, N. Engl. J. Med
Libster, Early high-titer plasma therapy to prevent severe covid-19 in older adults, N. Engl. J. Med
Luke, Kilbane, Jackson, Hoffman, Meta-analysis: Convalescent blood products for Spanish influenza pneumonia: A future H5N1 treatment?, Ann. Intern. Med
Mair-Jenkins, The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: A systematic review and exploratory meta-analysis, J. Infect. Dis
Marshall, A minimal common outcome measure set for COVID-19 clinical research, Lancet Infect. Dis
Self, Effect of hydroxychloroquine on clinical status at 14 days in hospitalized patients with COVID-19: A randomized clinical trial, JAMA
Simonovich, A randomized trial of convalescent plasma in Covid-19 severe pneumonia, N. Engl. J. Med
DOI record:
{
"DOI": "10.1038/s41598-022-19629-z",
"ISSN": [
"2045-2322"
],
"URL": "http://dx.doi.org/10.1038/s41598-022-19629-z",
"abstract": "<jats:title>Abstract</jats:title><jats:p>Passive immunotherapy with convalescent plasma may be the only available agent during the early phases of a pandemic. Here, we report safety and efficacy of high-titer convalescent plasma for COVID-19 pneumonia. Double-blinded randomized multicenter placebo-controlled trial of adult patients hospitalized with COVID-19 pneumonia. The intervention was COVID-19 convalescent plasma and placebo was saline allocated 2:1. The primary outcome was clinical status 14 days after the intervention evaluated on a clinical ordinal scale. The trial was registered at ClinicalTrials.Gov, NCT04345289, 14/04/2020. The CCAP-2 trial was terminated prematurely due to futility. Of 147 patients randomized, we included 144 patients in the modified intention-to-treat population. The ordinal clinical status 14 days post-intervention was comparable between treatment groups (odds ratio (OR) 1.41, 95% confidence interval (CI) 0.72–2.09). Results were consistent when evaluating clinical progression on an individual level 14 days after intervention (OR 1.09; 95% CI 0.46–1.73). No significant differences in length of hospital stay, admission to ICU, frequency of severe adverse events or all-cause mortality during follow-up were found between the intervention and the placebo group. Infusion of convalescent plasma did not influence clinical progression, survival or length of hospitalization in patients with COVID-19 pneumonia.</jats:p>",
"alternative-id": [
"19629"
],
"article-number": "16385",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "24 January 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "31 August 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "30 September 2022"
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1,
"value": "Dr Nielsen declares grants from Novo Nordisk Foundation and personal fees from GSK and MDS outside of the submitted work. Dr Østergaard reports personal fees from Sanofi. Dr Erikstrup reports grants from Abbots diagnostic outside of the submitted work. Dr Benfield reports grants from Novo Nordisk Foundation, grants from Simonsen Foundation, grants and personal fees from GSK, grants and personal fees from Pfizer, personal fees from Boehringer Ingelheim, personal fees from Abbvie, grants and personal fees from Gilead, personal fees from MSD, grants from Lundbeck Foundation, grants from Kai Hansen Foundation, and grants from Erik and Susanna Olsen’s Charitable Fund outside the submitted work. All other authors have nothing to disclose."
}
],
"author": [
{
"affiliation": [],
"family": "Thorlacius-Ussing",
"given": "Louise",
"sequence": "first"
},
{
"affiliation": [],
"family": "Brooks",
"given": "Patrick Terrence",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nielsen",
"given": "Henrik",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jensen",
"given": "Bitten Aagaard",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wiese",
"given": "Lothar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sækmose",
"given": "Susanne Gjørup",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Johnsen",
"given": "Stine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gybel-Brask",
"given": "Mikkel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Johansen",
"given": "Isik S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bruun",
"given": "Mie Topholm",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Stærke",
"given": "Nina Breinholdt",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Østergaard",
"given": "Lars",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Erikstrup",
"given": "Christian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ostrowski",
"given": "Sisse Rye",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Homburg",
"given": "Keld Mikkelsen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Georgsen",
"given": "Jørgen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mikkelsen",
"given": "Susan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sandholdt",
"given": "Håkon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Leding",
"given": "Cæcilie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hovmand",
"given": "Nichlas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Clausen",
"given": "Clara Lundetoft",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tinggaard",
"given": "Michaela",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pedersen",
"given": "Karen Brorup Heje",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Iversen",
"given": "Katrine Kjær",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tingsgård",
"given": "Sandra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Israelsen",
"given": "Simone Bastrup",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Benfield",
"given": "Thomas",
"sequence": "additional"
}
],
"container-title": "Scientific Reports",
"container-title-short": "Sci Rep",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2022,
9,
30
]
],
"date-time": "2022-09-30T11:15:12Z",
"timestamp": 1664536512000
},
"deposited": {
"date-parts": [
[
2022,
9,
30
]
],
"date-time": "2022-09-30T11:15:20Z",
"timestamp": 1664536520000
},
"funder": [
{
"DOI": "10.13039/501100003554",
"doi-asserted-by": "publisher",
"name": "Lundbeckfonden"
}
],
"indexed": {
"date-parts": [
[
2022,
10,
1
]
],
"date-time": "2022-10-01T05:16:49Z",
"timestamp": 1664601409930
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2022,
9,
30
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2022,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
9,
30
]
],
"date-time": "2022-09-30T00:00:00Z",
"timestamp": 1664496000000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
9,
30
]
],
"date-time": "2022-09-30T00:00:00Z",
"timestamp": 1664496000000
}
}
],
"link": [
{
"URL": "https://www.nature.com/articles/s41598-022-19629-z.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-022-19629-z",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-022-19629-z.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1038",
"published": {
"date-parts": [
[
2022,
9,
30
]
]
},
"published-online": {
"date-parts": [
[
2022,
9,
30
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.7326/0003-4819-145-8-200610170-00139",
"author": "TC Luke",
"doi-asserted-by": "publisher",
"first-page": "599",
"issue": "8",
"journal-title": "Ann. Intern. Med.",
"key": "19629_CR1",
"unstructured": "Luke, T. C., Kilbane, E. M., Jackson, J. L. & Hoffman, S. L. Meta-analysis: Convalescent blood products for Spanish influenza pneumonia: A future H5N1 treatment?. Ann. Intern. Med. 145(8), 599–609 (2006).",
"volume": "145",
"year": "2006"
},
{
"DOI": "10.1093/cid/ciq106",
"author": "IF Hung",
"doi-asserted-by": "publisher",
"first-page": "447",
"issue": "4",
"journal-title": "Clin. Infect. Dis.",
"key": "19629_CR2",
"unstructured": "Hung, I. F. et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin. Infect. Dis. 52(4), 447–456 (2011).",
"volume": "52",
"year": "2011"
},
{
"DOI": "10.1093/infdis/jiu396",
"author": "J Mair-Jenkins",
"doi-asserted-by": "publisher",
"first-page": "80",
"issue": "1",
"journal-title": "J. Infect. Dis.",
"key": "19629_CR3",
"unstructured": "Mair-Jenkins, J. et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: A systematic review and exploratory meta-analysis. J. Infect. Dis. 211(1), 80–90 (2015).",
"volume": "211",
"year": "2015"
},
{
"DOI": "10.1001/jama.2021.2747",
"author": "P Janiaud",
"doi-asserted-by": "publisher",
"first-page": "1185",
"issue": "12",
"journal-title": "JAMA",
"key": "19629_CR4",
"unstructured": "Janiaud, P. et al. Association of convalescent plasma treatment with clinical outcomes in patients with COVID-19: A systematic review and meta-analysis. JAMA 325(12), 1185–1195 (2021).",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(21)00897-7",
"author": "RECOVERY Collaborative Group",
"doi-asserted-by": "publisher",
"first-page": "2049",
"issue": "10289",
"journal-title": "Lancet Lond. Engl.",
"key": "19629_CR5",
"unstructured": "RECOVERY Collaborative Group. Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): A randomised controlled, open-label, platform trial. Lancet Lond. Engl. 397(10289), 2049–2059 (2021).",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1016/j.jbi.2019.103208",
"author": "PA Harris",
"doi-asserted-by": "publisher",
"journal-title": "J. Biomed. Inform.",
"key": "19629_CR6",
"unstructured": "Harris, P. A. et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 95, 103208 (2019).",
"volume": "95",
"year": "2019"
},
{
"DOI": "10.1016/j.jbi.2008.08.010",
"author": "PA Harris",
"doi-asserted-by": "publisher",
"first-page": "377",
"issue": "2",
"journal-title": "J. Biomed. Inform.",
"key": "19629_CR7",
"unstructured": "Harris, P. A. et al. Research electronic data capture (REDCap): A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 42(2), 377–381 (2009).",
"volume": "42",
"year": "2009"
},
{
"key": "19629_CR8",
"unstructured": "Georgsen, J., Bagge Hansen, M. & Sørensen, B. Transfusionsmedicinske Standarder. https://dski.dk/wp-content/uploads/2019/07/tms-5-0-2019.pdf (2019)."
},
{
"DOI": "10.1016/S1473-3099(20)30483-7",
"author": "JC Marshall",
"doi-asserted-by": "publisher",
"first-page": "e192",
"issue": "8",
"journal-title": "Lancet Infect. Dis.",
"key": "19629_CR9",
"unstructured": "Marshall, J. C. et al. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect. Dis. 20(8), e192–e197 (2020).",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2007764",
"author": "JH Beigel",
"doi-asserted-by": "publisher",
"first-page": "1813",
"issue": "19",
"journal-title": "N. Engl. J. Med.",
"key": "19629_CR10",
"unstructured": "Beigel, J. H. et al. Remdesivir for the treatment of Covid-19: Final report. N. Engl. J. Med. 383(19), 1813–1826 (2020).",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.22240",
"author": "WH Self",
"doi-asserted-by": "publisher",
"first-page": "2165",
"issue": "21",
"journal-title": "JAMA",
"key": "19629_CR11",
"unstructured": "Self, W. H. et al. Effect of hydroxychloroquine on clinical status at 14 days in hospitalized patients with COVID-19: A randomized clinical trial. JAMA 324(21), 2165–2176 (2020).",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2033700",
"author": "R Libster",
"doi-asserted-by": "publisher",
"first-page": "610",
"issue": "7",
"journal-title": "N. Engl. J. Med.",
"key": "19629_CR12",
"unstructured": "Libster, R. et al. Early high-titer plasma therapy to prevent severe covid-19 in older adults. N. Engl. J. Med. 384(7), 610–618 (2021).",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2103784",
"author": "FK Korley",
"doi-asserted-by": "publisher",
"first-page": "1951",
"journal-title": "N. Engl. J. Med.",
"key": "19629_CR13",
"unstructured": "Korley, F. K. et al. Early convalescent plasma for high-risk outpatients with Covid-19. N. Engl. J. Med. 385, 1951–1960 (2021).",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1172/JCI152740",
"author": "C Avendaño-Solá",
"doi-asserted-by": "publisher",
"first-page": "e152740",
"journal-title": "J Clin Invest.",
"key": "19629_CR14",
"unstructured": "Avendaño-Solá, C. et al. A multicenter randomized open-label clinical trial for convalescent plasma in patients hospitalized with COVID-19 pneumonia. J Clin Invest. 1231, e152740 (2021).",
"volume": "1231",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2031893",
"author": "MJ Joyner",
"doi-asserted-by": "publisher",
"first-page": "1015",
"issue": "11",
"journal-title": "N. Engl. J. Med.",
"key": "19629_CR15",
"unstructured": "Joyner, M. J. et al. Convalescent plasma antibody levels and the risk of death from Covid-19. N. Engl. J. Med. 384(11), 1015–1027 (2021).",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1136/bmj.m3939",
"author": "A Agarwal",
"doi-asserted-by": "publisher",
"journal-title": "BMJ",
"key": "19629_CR16",
"unstructured": "Agarwal, A. et al. Convalescent plasma in the management of moderate covid-19 in adults in India: Open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ 371, m3939 (2020).",
"volume": "371",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2031304",
"author": "VA Simonovich",
"doi-asserted-by": "publisher",
"first-page": "619",
"issue": "7",
"journal-title": "N. Engl. J. Med.",
"key": "19629_CR17",
"unstructured": "Simonovich, V. A. et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N. Engl. J. Med. 384(7), 619–629 (2021).",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1128/JCM.02596-20",
"author": "LH Harritshøj",
"doi-asserted-by": "publisher",
"first-page": "e02596",
"issue": "5",
"journal-title": "J. Clin. Microbiol.",
"key": "19629_CR18",
"unstructured": "Harritshøj, L. H. et al. Comparison of 16 serological SARS-CoV-2 immunoassays in 16 clinical laboratories. J. Clin. Microbiol. 59(5), e02596-e2620 (2021).",
"volume": "59",
"year": "2021"
}
],
"reference-count": 18,
"references-count": 18,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.nature.com/articles/s41598-022-19629-z"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Multidisciplinary"
],
"subtitle": [],
"title": "A randomized placebo-controlled trial of convalescent plasma for adults hospitalized with COVID-19 pneumonia",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "12"
}