An investigation into the Effects of Intravenous Vitamin C on Pulmonary CT Findings and Clinical Outcomes of Patients with COVID 19 Pneumonia A Randomized Clinical Trial
et al., Urology Journal, doi:10.22037/uj.v18i.6863, Nov 2021
Vitamin C for COVID-19
6th treatment shown to reduce risk in
September 2020, now with p = 0.000000068 from 74 studies, recognized in 22 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
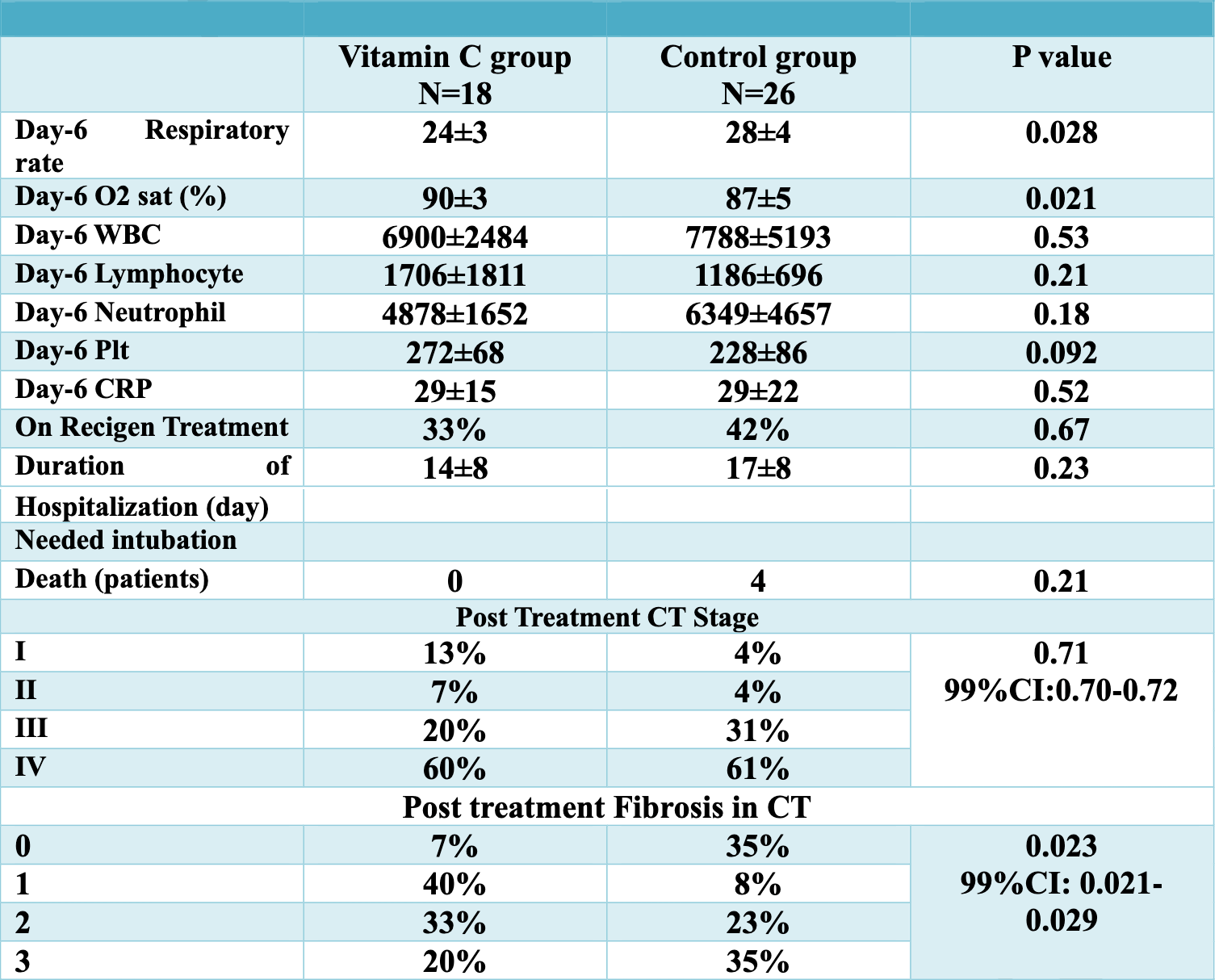
RCT 54 late stage patients, 18 treated with IV vitamin C (2g every 6h for 5 days), showing significant relative improvements in oxygen saturation and respiratory rate.
This is the 8th of 21 COVID-19 RCTs for vitamin C, which collectively show efficacy with p=0.0012.
This is the 35th of 74 COVID-19 controlled studies for vitamin C, which collectively show efficacy with p=0.000000068.
|
risk of death, 87.1% lower, RR 0.13, p = 0.13, treatment 0 of 18 (0.0%), control 4 of 26 (15.4%), NNT 6.5, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
hospitalization time, 17.6% lower, relative time 0.82, p = 0.23, treatment 18, control 26.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Tehrani et al., 8 Nov 2021, Randomized Controlled Trial, Iran, peer-reviewed, 10 authors, study period March 2020 - May 2020, average treatment delay 9.0 days, dosage 2000mg qid days 1-5.
Contact: saraabolghasemi1@gmail.com.
An investigation into the effects of intravenous vitamin C on pulmonary CT findings and clinical outcomes of patients with COVID 19 pneumonia A Randomized Clinical Trial
Purpose:In late December 2019, a series of unexplained cases of pneumonia were reported in Wuhan, China. On January 12, 2020, the World Health Organization temporarily named the virus responsible for the emerging cases of pneumonia as the 2019 coronavirus. Acute respiratory distress syndrome (ARDS) due to Covid-19 has rapidly spread around the world, and while no specific treatment or vaccine has been reported, mortality rates remain high. One of the suggested treatments for cellular damage in the pathogenesis of ARDS caused by the coronavirus is the administration of high doses of intravenous vitamin C. Considering the paucity of literature on the therapeutic effects of high doses of intravenous vitamin C in patients with ARDS resulting from the coronavirus, this study was conducted to assess this therapeutic supplement in these patients.
Materials and Methods: This study was performed as a single-center clinical trial in patients with a documented diagnosis of COVID-19 pneumonia. 54 eligible patients with moderate to severe COVID-19 symptoms, based on specific inclusion and exclusion criteria, were included in the investigation and randomly divided into two groups. The control group consisted of 26 patients who received standard treatment, whereas the treatment group was comprised of 18 patients administered intravenous vitamin C at a dose of 2 g every 6 hours for 5 days in addition to standard treatment. Demographic characteristics, underlying diseases, length of hospital stay, and mortality rates were reviewed and collected. Oxygen saturation, respiratory rates, serum C Reactive Protein (CRP) levels, lymphopenia and lung parenchymal involvement on CT were investigated at the time of admission and on the sixth day after hospitalization. Finally, all variables were analyzed with IBM SPSS Statistics 23 software and a significant statistical difference was defined for all variables, P <0.05. Results: Of these variables, the amount of oxygen saturation in the vitamin C group increased significantly from 86±5% on the first day of hospitalization to 90±3% on the sixth day of hospitalization (P value=0.02). Also, the respiratory rate in the vitamin C group decreased significantly from 27±3 on the first day of hospitalization to 24±3 on the sixth day of hospitalization (P value=0.03). Lung CT scans of patients in the two groups reported by two radiologists were also compared. Based on the report of the radiologists, the rate of lung involvement in the vitamin C group was significantly lower than in the control group at the end of treatment (P value=0.02). Conclusions: Due to the effectiveness of high doses of intravenous vitamin C on reducing lung involvement and improving clinical symptoms, further studies with a larger sample size are recommended to demonstrate the effects of this drug supplement.
CONFLICT OF INTERESTS The authors did not declare any conflict of interest. Table 2 . For classifying lung zone involvement, three-zone were defined as follows: upper zone: above the carina region, middle zone: the area between the carina and inferior pulmonary vein, and lower zone: below the inferior pulmonary vein.
Lung involvement scoring Definition
References
Carr, Maggini, Vitamin, Immune Function, Nutrients, doi:10.3390/nu9111211PMID:29099763
Cheng, Can early and high intravenous dose of vitamin C prevent and treat coronavirus disease 2019 (COVID-19)?, Med Drug Discov
Englard, Seifter, The biochemical functions of ascorbic acid, Annu RevNutr, doi:10.1146/annurev.nu.06.070186.002053
Fowler, Iii, None
Hakamifard, Soltani, Maghsoudi, The effect of vitamin E and vitamin C in patients with COVID-19 pneumonia; a randomized controlled clinical trial, Immunopathol Persa
Hemilä, Vitamin C and Infections, Nutrients
Hosseini Zabet, Mohammadi, Ramezani, Khalili, Effect of high-dose Ascorbic acid on vasopressor's requirement in septic shock, J Res Pharm Pract
Jamalimoghadamsiahkali, Zarezade, Koolaji, Seyedalinaghi, Zendehdel et al., Safety and effectiveness of high-dose vitamin C in patients with COVID-19: A randomized open-label clinical trial, Eur. J. Med. Res
Kindler, Thiel, SARS-CoV and IFN: Too Little, Too Late Cell Host Microbe
Maggini, Wintergerst, Beveridge, Hornig, Selected vitamins and trace elements to support immune function by strengthening epithelial barriers and cellular and humoral immune responses, Br. J. Nutr
Mandl, Szarka, Ba´nhegyi, Vitamin C: update on physiology and pharmacology, Br J Pharmacol, doi:10.1111/j.1476
Natarajan, Brophy, Effect of Vitamin C Infusion on Organ Failure and Biomarkers of Inflammation and Vascular Injury in Patients with Sepsis and Severe Acute Respiratory Failure: The CITRIS-ALI Randomized Clinical Trial, JAMA
Parkin, Cohen, An overview of the immune system, Lancet
Syed, Knowlson, Sculthorpe, Farthing, Dewilde et al., Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis, J. Transl. Med
Truwit, Hite, Morris, Dewilde, Priday et al., None
Webb, Villamor, Update: Effects of antioxidant and non-antioxidant vitamin supplementation on immune function, Nutr. Rev
Wei, Wang, Liao, Guo, Wen et al., Efficacy of vitamin C in patients with sepsis:An updated meta-analysis, Eur. J. Pharmacol
Yoshikawa, Hill, Li, Peters, Tseng, Severe acute respiratory syndrome (SARS) coronavirus-induced lung epithelial cytokines exacerbate SARS pathogenesis by modulating intrinsic functions of macrophages and dendritic cells, J Virol
Zabetakis, Lordan, Norton, Tsoupras, COVID-19: The Inflammation Link and the Role of Nutrition in Potential Mitigation, Nutrients
Zhang, Rao, Li, Zhu, Liu et al., Pilot trial of high-dose vitamin C in critically ill COVID-19 patients, Ann. Intensive Care
DOI record:
{
"DOI": "10.22037/uj.v18i.6863",
"ISSN": "1735546, 17351308",
"URL": "https://doi.org/10.22037/uj.v18i.6863",
"abstract": "Purpose:In late December 2019, a series of unexplained cases of pneumonia were reported in Wuhan, China. On January 12, 2020, the World Health Organization temporarily named the virus responsible for the emerging cases of pneumonia as the 2019 coronavirus. Acute respiratory distress syndrome (ARDS) due to Covid-19 has rapidly spread around the world, and while no specific treatment or vaccine has been reported, mortality rates remain high. One of the suggested treatments for cellular damage in the pathogenesis of ARDS caused by the coronavirus is the administration of high doses of intravenous vitamin C. Considering the paucity of literature on the therapeutic effects of high doses of intravenous vitamin C in patients with ARDS resulting from the coronavirus, this study was conducted to assess this therapeutic supplement in these patients. Materials and Methods: This study was performed as a single-center clinical trial in patients with a documented diagnosis of COVID-19 pneumonia. 54 eligible patients with moderate to severe COVID-19 symptoms, based on specific inclusion and exclusion criteria, were included in the investigation and randomly divided into two groups. The control group consisted of 26 patients who received standard treatment, whereas the treatment group was comprised of 18 patients administered intravenous vitamin C at a dose of 2 g every 6 hours for 5 days in addition to standard treatment. Demographic characteristics, underlying diseases, length of hospital stay, and mortality rates were reviewed and collected. Oxygen saturation, respiratory rates, serum C Reactive Protein (CRP) levels, lymphopenia and lung parenchymal involvement on CT were investigated at the time of admission and on the sixth day after hospitalization. Finally, all variables were analyzed with IBM SPSS Statistics 23 software and a significant statistical difference was defined for all variables, P <0.05. Results: Of these variables, the amount of oxygen saturation in the vitamin C group increased significantly from 86±5% on the first day of hospitalization to 90±3% on the sixth day of hospitalization (P value=0.02). Also, the respiratory rate in the vitamin C group decreased significantly from 27±3 on the first day of hospitalization to 24±3 on the sixth day of hospitalization (P value=0.03). Lung CT scans of patients in the two groups reported by two radiologists were also compared. Based on the report of the radiologists, the rate of lung involvement in the vitamin C group was significantly lower than in the control group at the end of treatment (P value=0.02). Conclusion: Due to the effectiveness of high doses of intravenous vitamin C on reducing lung involvement and improving clinical symptoms, further studies with a larger sample size are recommended to demonstrate the effects of this drug supplement.",
"author": [
{
"family": "Tehrani",
"given": "Shabnam"
},
{
"family": "Yadegarynia",
"given": "Davood"
},
{
"family": "Abrishami",
"given": "Alireza"
},
{
"family": "Moradi",
"given": "Hamideh"
},
{
"family": "Gharaei",
"given": "Babak"
},
{
"family": "Rauofi",
"given": "Masoomeh"
},
{
"family": "Maghsoudi Nejad",
"given": "Fatemeh"
},
{
"family": "Sali",
"given": "Shahnaz"
},
{
"family": "Khabiri",
"given": "Neda"
},
{
"family": "Abolghasemi",
"given": "Sara"
}
],
"container-title": "Urology Journal",
"issue": "Instant 2021",
"issued": {
"date-parts": [
[
2021,
11,
8
]
]
},
"language": "eng",
"medium": "JB",
"page": "6863",
"page-first": "6863",
"publisher": "Urology and Nephrology Research Center, Shahid Beheshti University of Medical Sciences",
"publisher-place": "IR",
"title": "An investigation into the Effects of Intravenous Vitamin C on Pulmonary CT Findings and Clinical Outcomes of Patients with COVID 19 Pneumonia A Randomized Clinical Trial",
"type": "article-journal"
}
