Real-world clinical outcomes of treatment with casirivimab-imdevimab among patients with mild-to-moderate coronavirus disease 2019 during the Delta variant pandemic
et al., medRxiv, doi:10.1101/2021.12.19.21268078, Dec 2021
19th treatment shown to reduce risk in
March 2021, now with p = 0.000095 from 34 studies, recognized in 52 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 949 patients in Japan, 314 treated with casirivimab/imdevimab showing significantly lower risk of deterioration with treatment.
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for many omicron variants1-7.
Standard of Care (SOC) for COVID-19 in the study country,
Japan, is very poor with very low average efficacy for approved treatments8.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 200.0% higher, RR 3.00, p = 1.00, treatment 1 of 222 (0.5%), control 0 of 222 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm), propensity score matching.
|
|
risk of death, 59.6% lower, RR 0.40, p = 0.67, treatment 1 of 314 (0.3%), control 5 of 635 (0.8%), NNT 213, unadjusted.
|
|
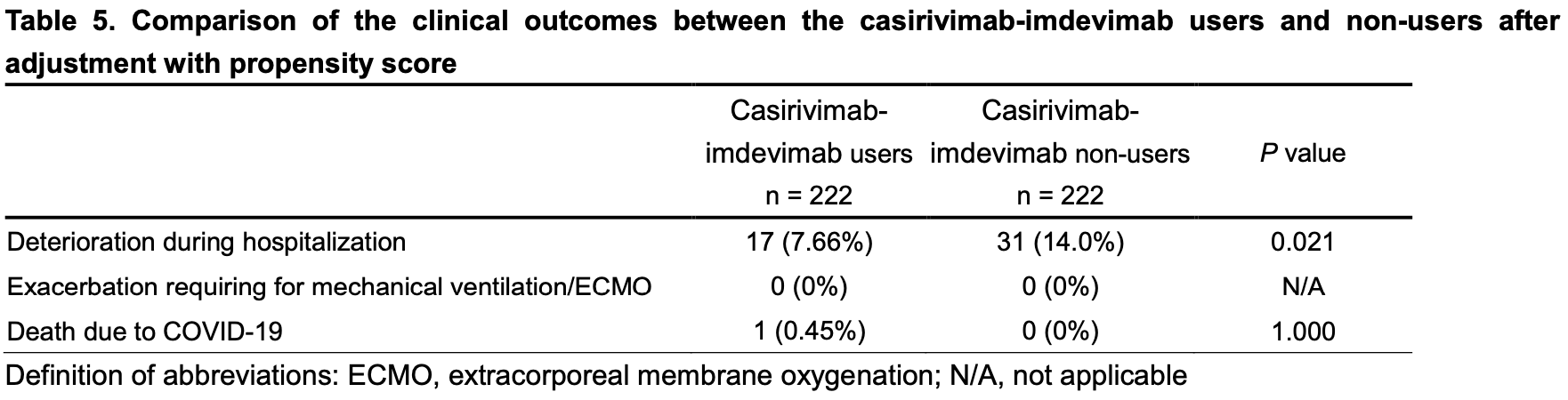
risk of progression, 45.2% lower, RR 0.55, p = 0.02, treatment 17 of 222 (7.7%), control 31 of 222 (14.0%), NNT 16, propensity score matching.
|
|
risk of progression, 49.9% lower, RR 0.50, p = 0.002, treatment 34 of 314 (10.8%), control 70 of 365 (19.2%), NNT 12, odds ratio converted to relative risk, multivariate.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
4.
Tatham et al., Lack of Ronapreve (REGN-CoV; casirivimab and imdevimab) virological efficacy against the SARS-CoV 2 Omicron variant (B.1.1.529) in K18-hACE2 mice, bioRxiv, doi:10.1101/2022.01.23.477397.
5.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
6.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
Suzuki et al., 21 Dec 2021, retrospective, Japan, preprint, 49 authors, study period 24 July, 2021 - 30 September, 2021.
Contact: shibatay@fmu.ac.jp.
Real-world clinical outcomes of treatment with casirivimab-imdevimab among patients with mild-to-moderate coronavirus disease 2019 during the Delta variant pandemic
doi:10.1101/2021.12.19.21268078
This real-world retrospective study demonstrates the contribution of treatment with casirivimab-imdevimab to the prevention of deterioration in patients with mild-to-All rights reserved. No reuse allowed without permission. perpetuity.
References
Abu-Raddad, Chemaitelly, Butt, Effectiveness of the BNT162b2 Covid-19 Vaccine against the B.1.1.7 and B.1.351 Variants, The New England journal of medicine
Austin, Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies, Pharmaceutical statistics
Baum, Fulton, Wloga, Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies, Science
Bernal, Andrews, Gower, Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant, The New England journal of medicine
Ganesh, Philpot, Bierle, Real-World Clinical Outcomes of Bamlanivimab and Casirivimab-Imdevimab Among High-Risk Patients With Mild to Moderate Coronavirus Disease 2019, The Journal of infectious diseases
Gottlieb, Nirula, Chen, Effect of Bamlanivimab as Monotherapy or in Combination With Etesevimab on Viral Load in Patients With Mild to Moderate COVID-19: A Randomized Clinical Trial, Jama
Hu, Peng, Wang, Emerging SARS-CoV-2 variants reduce neutralization sensitivity to convalescent sera and monoclonal antibodies, Cellular & molecular immunology
Huang, Yang, Xu, Xu, Liu, Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19, Acta pharmacologica Sinica
Ito, Piantham, Nishiura, Predicted dominance of variant Delta of SARS-CoV-2 before Tokyo Olympic Games, Japan, Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin
Korber, Fischer, Gnanakaran, Tracking Changes in SARS-CoV-2 Spike: Evidence that D614G Increases Infectivity of the COVID-19 Virus, Cell
Kumar, Banu, Sasikala, Effectiveness of REGEN-COV antibody cocktail against the B.1.617.2 (delta) variant of SARS-CoV-2: A cohort study, Journal of internal medicine
Planas, Veyer, Baidaliuk, Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization, Nature
Polack, Thomas, Kitchin, Safety and Efficacy of the BNT162b2 mRNA Covid-19
Razonable, Pawlowski, Horo, Casirivimab-Imdevimab treatment is associated with reduced rates of hospitalization among high-risk patients with mild to moderate coronavirus disease-19, EClinicalMedicine
Shiba, Kawahara, Using Propensity Scores for Causal Inference: Pitfalls and Tips, Journal of epidemiology
Shibata, Minemura, Suzuki, Development and external validation of the DOAT and DOATS scores: simple decision support tools to identify disease progression among nonelderly patients with mild/moderate COVID-19
Taylor, Adams, Hufford, De La Torre, Winthrop et al., Neutralizing monoclonal antibodies for treatment of COVID-19, Nature reviews Immunology
The_Official_Website_Of_Fukushima_Prefecture, Confirmed cases of COVID-19 in Fukushima
The_Official_Website_Of_Fukushima_Prefecture, The variant of COVID-19 in Fukushima from
Uriu, Kimura, Shirakawa, Neutralization of the SARS-CoV-2 Mu Variant by Convalescent and Vaccine Serum, The New England journal of medicine
Vaccine, None, The New England journal of medicine
Verderese, Stepanova, Lam, Neutralizing Monoclonal Antibody Treatment Reduces Hospitalization for Mild and Moderate COVID-19: A Real-World Experience, Clinical infectious diseases
Wang, Nair, Liu, Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7, Nature
Weinreich, Sivapalasingam, Norton, REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19, The New England journal of medicine
Wilhelm, Toptan, Pallas, Antibody-Mediated Neutralization of Authentic SARS-CoV
Yamamoto, Wada, Ichikawa, Evaluation of Biomarkers of Severity in Patients with COVID-19 Infection, J Clin Med
DOI record:
{
"DOI": "10.1101/2021.12.19.21268078",
"URL": "http://dx.doi.org/10.1101/2021.12.19.21268078",
"abstract": "<jats:p>Background: Mutations of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) may reduce the efficacy of neutralizing monoclonal antibody therapy against coronavirus disease 2019 (COVID-19). We here evaluated the efficacy of casirivimab-imdevimab in patients with mild-to-moderate COVID-19 during the Delta variant surge in Fukushima Prefecture, Japan.\nMethods: We enrolled 949 patients with mild-to-moderate COVID-19 who were admitted to hospital between July 24, 2021 and September 30, 2021. Clinical deterioration after admission was compared between casirivimab-imdevimab users (n = 314) and non-users (n = 635). \nResults: The casirivimab-imdevimab users were older (P < 0.0001), had higher body temperature (≥ 38 degree) (P < 0.0001) and greater rates of history of cigarette smoking (P = 0.0068), hypertension (P = 0.0004), obesity (P < 0.0001), and dyslipidemia (P < 0.0001) than the non-users. Multivariate logistic regression analysis demonstrated that receiving casirivimab-imdevimab was an independent factor for preventing deterioration (odds ratio 0.448; 95% confidence interval 0.263 to 0.763; P = 0.0023). Furthermore, in 222 patients who were selected from each group after matching on the propensity score, deterioration was significantly lower among those receiving casirivimab-imdevimab compared to those not receiving casirivimab-imdevimab (7.66% vs 14.0%; p = 0.021).\nConclusion: This real-world study demonstrates that casirivimab-imdevimab contributes to the prevention of deterioration in COVID-19 patients after hospitalization during a Delta variant surge.</jats:p>",
"accepted": {
"date-parts": [
[
2021,
12,
21
]
]
},
"author": [
{
"affiliation": [],
"family": "Suzuki",
"given": "Yasuhito",
"sequence": "first"
},
{
"affiliation": [],
"family": "Shibata",
"given": "Yoko",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Minemura",
"given": "Hiroyuki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nikaido",
"given": "Takehumi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tanino",
"given": "Yoshinori",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fukuhara",
"given": "Atsuro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kanno",
"given": "Ryuzo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saito",
"given": "Hiroyuki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Suzuki",
"given": "Shuzo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ishii",
"given": "Taeko",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Inokoshi",
"given": "Yayoi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sando",
"given": "Eiichiro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sakuma",
"given": "Hirofumi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kobayashi",
"given": "Tatsuho",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kume",
"given": "Hiroaki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kamimoto",
"given": "Masahiro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aoki",
"given": "Hideko",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Takama",
"given": "Akira",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kamiyama",
"given": "Takamichi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nakayama",
"given": "Masaru",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saito",
"given": "Kiyoshi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tanigawa",
"given": "Koichi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sato",
"given": "Masahiko",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kanbe",
"given": "Toshiyuki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kanzaki",
"given": "Norio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Azuma",
"given": "Teruhisa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sakamoto",
"given": "Keiji",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nakamura",
"given": "Yuichi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Otani",
"given": "Hiroshi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Waragai",
"given": "Mitsuru",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Maeda",
"given": "Shinsaku",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ishida",
"given": "Tokiya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sugino",
"given": "Keishi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tsukada",
"given": "Yasuhiko",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yamada",
"given": "Ryuki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sato",
"given": "Riko",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Omuna",
"given": "Takumi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tomita",
"given": "Hikaru",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saito",
"given": "Mikako",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Watanabe",
"given": "Natsumi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rikimaru",
"given": "Mami",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kawamata",
"given": "Takaya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Umeda",
"given": "Takashi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Morimoto",
"given": "Julia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Togawa",
"given": "Ryuichi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sato",
"given": "Yuki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saito",
"given": "Junpei",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kanazawa",
"given": "Kenya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Iseki",
"given": "Ken",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
12,
22
]
],
"date-time": "2021-12-22T03:45:14Z",
"timestamp": 1640144714000
},
"deposited": {
"date-parts": [
[
2021,
12,
22
]
],
"date-time": "2021-12-22T03:45:14Z",
"timestamp": 1640144714000
},
"group-title": "Infectious Diseases (except HIV/AIDS)",
"indexed": {
"date-parts": [
[
2021,
12,
22
]
],
"date-time": "2021-12-22T06:54:21Z",
"timestamp": 1640156061532
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2021,
12,
21
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2021.12.19.21268078",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2021,
12,
21
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2021,
12,
21
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference-count": 0,
"references-count": 0,
"relation": {},
"score": 1,
"short-container-title": [],
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": [
"Real-world clinical outcomes of treatment with casirivimab-imdevimab among patients with mild-to-moderate coronavirus disease 2019 during the Delta variant pandemic"
],
"type": "posted-content"
}
