Non-linear oral bioavailability and clinical pharmacokinetics of high-dose Andrographis paniculata ethanolic extract: relevant dosage implications for COVID-19 treatment
et al., Pharmaceutical Biology, doi:10.1080/13880209.2024.2444446, TCTR20210201005, Jan 2025
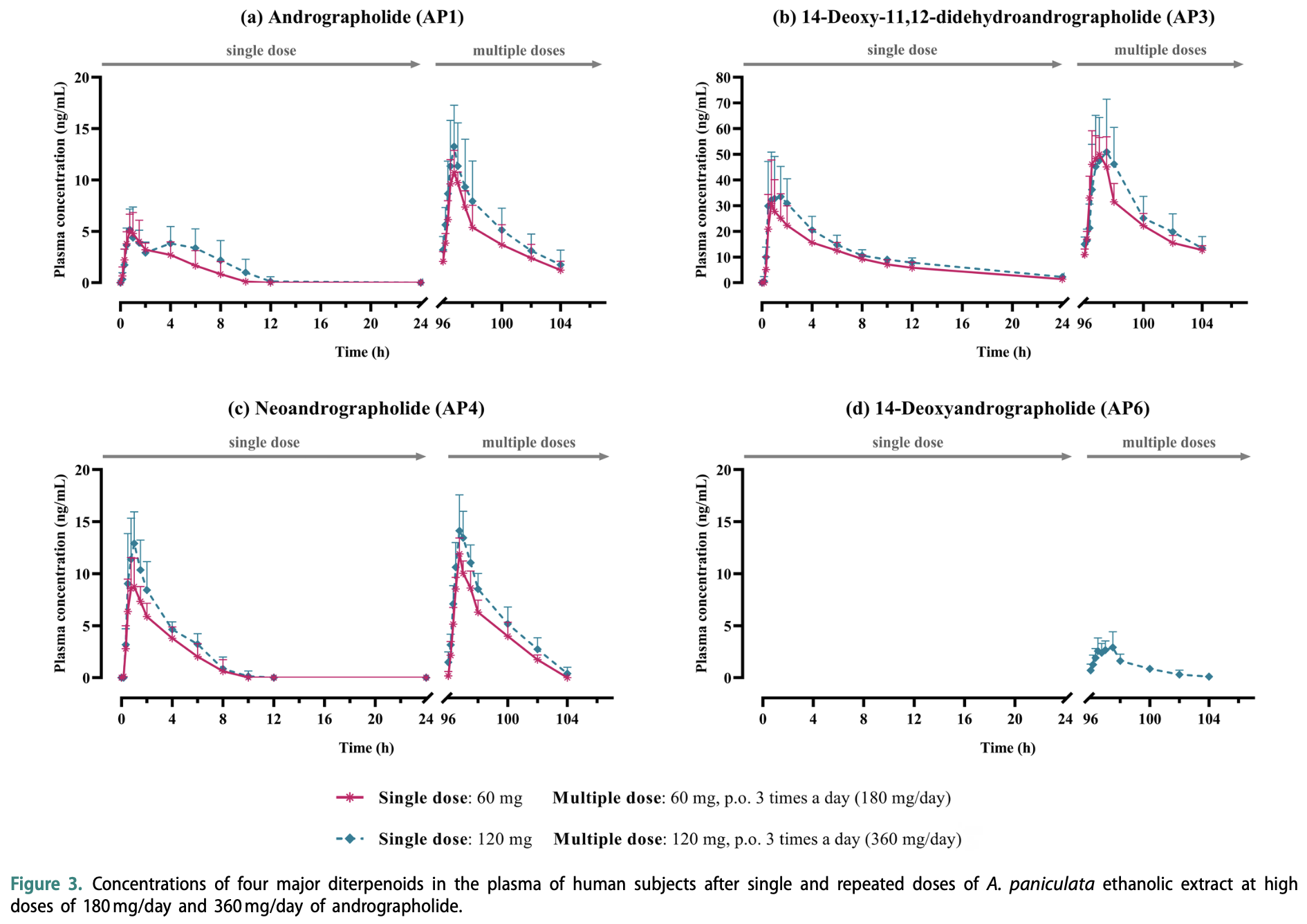
Analysis of the pharmacokinetics and safety of high-dose Andrographis paniculata ethanolic extract. Authors observed non-linear oral bioavailability, with low plasma concentrations of key bioactive diterpenoids following ethanolic extract doses equivalent to 180 mg/day and 360 mg/day of andrographolide. Safety analysis showed mild and transient adverse events, with no significant clinical concerns. Authors suggest that the current ethanolic extraction method may impair bioavailability and recommend further research on alternative extraction methods or formulations to enhance bioavailability. Purified andrographolide may offer greater effectiveness due to limitations of the extract such as residual components like waxes or resins hindering release and absorption.
Songvut et al., 6 Jan 2025, Thailand, peer-reviewed, 8 authors, trial TCTR20210201005.
Contact: tawit@cri.or.th, jutamaad@cri.or.th.
Non-linear oral bioavailability and clinical pharmacokinetics of high-dose Andrographis paniculata ethanolic extract: relevant dosage implications for COVID-19 treatment
Pharmaceutical Biology, doi:10.1080/13880209.2024.2444446
Aim: insufficient quality control and limited dissolution of Andrographis paniculata extract capsules restricts their bioavailability and hinder the clinical use for treating mild coronavirus disease 2019 (cOViD-19) patients. Objective: this study aims to investigate pharmacokinetics and safety of high-dosage A. paniculata ethanolic extract (equivalent to 180 or 360 mg/day of andrographolide), relevant dosages used for mild cOViD-19 treatment. Methods: an open-label, single-dose, and repeated-dose conducted in healthy volunteers. subjects received capsules containing ethanolic extract equivalent to andrographolide dosage of either 60 or 120 mg per dose, taken every eight hours daily (totaling 180 or 360 mg/day). safety was assessed through blood chemical analysis and adverse event monitoring after 7 days of ethanolic extract administration. Results: Pharmacokinetics of ethanolic extract indicated low plasma levels of the major diterpenoids. the maximum plasma concentration (cmax) of andrographolide did not exhibit a dose-proportional increase, reaching 6.44 and 11.62 µg/l for single and repeated doses of 60 mg/day, respectively. Doubling the dose (120 mg/day) only resulted in slightly higher cmax (6.97 and 15.03 µg/l for single and repeated doses, respectively). safety evaluation revealed mild, transient adverse events, but all parameters remained within normal ranges. Conclusions: this study highlights limitations in the pharmacokinetics of the ethanolic extract of A. paniculata. it indicated non-linear proportionality in the oral bioavailability of andrographolide. these findings suggest that current extraction process of ethanolic extract may hinder its effectiveness. Further research is warranted to explore alternative extraction methods or formulation developments that can enhance the bioavailability of andrographolide and its potential therapeutic effects for cOViD-19 treatment. TRIAL REGISTRATION the trial "safety and pharmacokinetic studies of Andrographis paniculata extracts in thai healthy volunteers" (tctR20210201005) was registered on thaiclinicaltrials.org (trial URl: https://www.thaiclinicaltrials.org/ export/pdf/ tctR20210201005) in accordance with the WhO international clinical trials Registry Platform (WhO-ictRP). the initial registration date was February 1, 2021, with the first subject recruited on November 1, 2021.
Author contributions Concept development, P.S., T.S., N.R., D.P., P.P. and J.S.; Clinical research, P.S. and P.P.; Herbal product analysis, N.P. and N.R.; Sample preparation, J.A.; Sample analysis and data curation, P.S. and T.S.; Pharmacokinetic and statistical analysis, P.S.; Writing original manuscript, P.S., J.A. and T.S.; Review manuscript, T.S., N.R., D.P., P.P. and J.S.
Disclosure statement The authors declare that there is no known conflict of interest with any organization regarding the materials discussed in the manuscript.
References
Abubakar, Haque, Preparation of medicinal plants: basic extraction and fractionation procedures for experimental purposes, J Pharm Bioallied Sci, doi:10.4103/jpbs.JPBS_175_19
Benjaponpitak, Sawaengtham, Thaneerat, Wanaratna, Chotsiri et al., Effect of Andrographis paniculata treatment for patients with early-stage COVID-19 on the prevention of pneumonia: a retrospective cohort study, Arch. Intern. Med. Res. medRxiv
Bitwell, Indra, Luke, Kakoma, A review of modern and conventional extraction techniques and their applications for extracting phytochemicals from plants, Sci Afr, doi:10.1016/j.sciaf.2023.e01585
Chien, Liu, Chang, Fang, Pai et al., Therapeutic effects of herbal-medicine combined therapy for COVID-19: a systematic review and meta-analysis of randomized controlled trials, Front Pharmacol, doi:10.3389/fphar.2022.950012
Damasceno, Da Rosa, De Araújo, Cardoso Furtado, Andrographis paniculata formulations: impact on diterpene lactone oral bioavailability, Eur J Drug Metab Pharmacokinet, doi:10.1007/s13318-021-00736-7
Fuzimoto, Isidoro, The antiviral and coronavirus-host protein pathways inhibiting properties of herbs and natural compoundsadditional weapons in the fight against the COVID-19 pandemic?, J Tradit Complement Med, doi:10.1016/j.jtcme.2020.05.003
Isidoro, Chang, Sheen, Natural products as a source of novel drugs for treating SARS-CoV2 infection, J Tradit Complement Med, doi:10.1016/j.jtcme.2022.02.001
Kaewdech, Nawalerspanya, Assawasuwannakit, Chamroonkul, Jandee et al., The use of Andrographis paniculata and its effects on liver biochemistry of patients with gastrointestinal problems in Thailand during the COVID-19 pandemic: a cross-sectional study, Sci Rep, doi:10.1038/s41598-022-23189-7
Lim, Chan, Tan, Teh, Abd Razak et al., Andrographis paniculata (Burm. f.) Wall. ex Nees, andrographolide, and andrographolide analogues as SARS-CoV-2 antivirals? A rapid review, Nat Prod Commun
Msemburi, Karlinsky, Knutson, Aleshin-Guendel, Chatterji et al., The WHO estimates of excess mortality associated with the COVID-19 pandemic, Nature, doi:10.1038/s41586-022-05522-2
Pholphana, Panomvana, Rangkadilok, Suriyo, Puranajoti et al., Andrographis paniculata: dissolution investigation and pharmacokinetic studies of four major active diterpenoids after multiple oral dose administration in healthy Thai volunteers, J Ethnopharmacol, doi:10.1016/j.jep.2016.09.058
Pholphana, Rangkadilok, Saehun, Ritruechai, Satayavivad, Changes in the contents of four active diterpenoids at different growth stages in Andrographis paniculata (Burm.f.) Nees (Chuanxinlian), Chin Med, doi:10.1186/1749-8546-8-2
Rangkadilok, Pholphana, Akanimanee, Panomvana, Puranajoti et al., Comparison of diterpenoid contents and dissolution profiles of selected Andrographis paniculata crude and extract capsules, Phytochem Anal, doi:10.1002/pca.3364
Ratiani, Pachkoria, Mamageishvili, Shengelia, Hovhannisyan et al., Efficacy of Kan Jang(®) in patients with mild COVID-19: interim analysis of a randomized, quadruple-blind, placebo-controlled trial, Pharmaceuticals, doi:10.3390/ph15081013
Sa-Ngiamsuntorn, Suksatu, Pewkliang, Thongsri, Kanjanasirirat et al., Anti-SARS-CoV-2 activity of Andrographis paniculata extract and its major component andrographolide in human lung epithelial cells and cytotoxicity evaluation in major organ cell representatives, J Nat Prod, doi:10.1021/acs.jnatprod.0c01324
Sakpal, Sample size estimation in clinical trial, Perspect Clin Res, doi:10.4103/2229-3485.71856
Sermkaew, Ketjinda, Boonme, Phadoongsombut, Wiwattanapatapee, Liquid and solid self-microemulsifying drug delivery systems for improving the oral bioavailability of andrographolide from a crude extract of Andrographis paniculata, Eur J Pharm Sci, doi:10.1016/j.ejps.2013.08.006
Siripongboonsitti, Ungtrakul, Tawinprai, Auewarakul, Chartisathian et al., Efficacy of Andrographis paniculata extract treatment in mild to moderate COVID-19 patients being treated with favipiravir: a double-blind, randomized, placebo-controlled study (APFaVi trial), Phytomedicine, doi:10.1016/j.phymed.2023.155018
Songvut, Boonyarattanasoonthorn, Nuengchamnong, Junsai, Kongratanapasert et al., Enhancing oral bioavailability of andrographolide using solubilizing agents and bioenhancer: comparative pharmacokinetics of Andrographis paniculata formulations in beagle dogs, Pharm Biol, doi:10.1080/13880209.2024.2311201
Songvut, Pholphana, Suriyo, Rangkadilok, Panomvana et al., A validated LC-MS/MS method for clinical pharmacokinetics and presumptive phase II metabolic pathways following oral administration of Andrographis paniculata extract, Sci Rep, doi:10.1038/s41598-023-28612-1
Songvut, Rangkadilok, Pholphana, Suriyo, Panomvana et al., Comparative pharmacokinetics and safety evaluation of high dosage regimens of Andrographis paniculata aqueous extract after single and multiple oral administration in healthy participants, Front Pharmacol, doi:10.3389/fphar.2023.1230401
Songvut, Suriyo, Panomvana, Rangkadilok, Satayavivad, A comprehensive review on disposition kinetics and dosage of oral administration of Andrographis paniculata, an alternative herbal medicine, in co-treatment of coronavirus disease, Front Pharmacol, doi:10.3389/fphar.2022.952660
Wanaratna, Leethong, Inchai, Chueawiang, Sriraksa et al., Andrographis paniculata extract in patients with mild COVID-19: a randomized controlled trial, Arch Intern Med Res, doi:10.26502/aimr.0125
Zou, Ding, Huang, Yang, Li et al., Andrographolide/phospholipid/cyclodextrin complex-loaded nanoemulsion: preparation, optimization, in vitro and in vivo evaluation, Biol Pharm Bull, doi:10.1248/bpb.b22-00154
DOI record:
{
"DOI": "10.1080/13880209.2024.2444446",
"ISSN": [
"1388-0209",
"1744-5116"
],
"URL": "http://dx.doi.org/10.1080/13880209.2024.2444446",
"alternative-id": [
"10.1080/13880209.2024.2444446"
],
"assertion": [
{
"label": "Peer Review Statement",
"name": "peerreview_statement",
"order": 1,
"value": "The publishing and review policy for this title is described in its Aims & Scope."
},
{
"URL": "http://www.tandfonline.com/action/journalInformation?show=aimsScope&journalCode=iphb20",
"label": "Aim & Scope",
"name": "aims_and_scope_url",
"order": 2,
"value": "http://www.tandfonline.com/action/journalInformation?show=aimsScope&journalCode=iphb20"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2024-07-18"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Revised",
"name": "revised",
"order": 1,
"value": "2024-11-20"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "2024-12-14"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 3,
"value": "2025-01-06"
}
],
"author": [
{
"affiliation": [
{
"name": "Laboratory of Pharmacology, Chulabhorn Research Institute, Bangkok, Thailand"
},
{
"name": "Center of Excellence on Environmental Health and Toxicology (EHT), OPS, MHESI, Bangkok, Thailand"
}
],
"family": "Songvut",
"given": "Phanit",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Laboratory of Pharmacology, Chulabhorn Research Institute, Bangkok, Thailand"
}
],
"family": "Akanimanee",
"given": "Jaratluck",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Laboratory of Pharmacology, Chulabhorn Research Institute, Bangkok, Thailand"
},
{
"name": "Center of Excellence on Environmental Health and Toxicology (EHT), OPS, MHESI, Bangkok, Thailand"
}
],
"family": "Suriyo",
"given": "Tawit",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Laboratory of Pharmacology, Chulabhorn Research Institute, Bangkok, Thailand"
}
],
"family": "Pholphana",
"given": "Nanthanit",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Laboratory of Pharmacology, Chulabhorn Research Institute, Bangkok, Thailand"
},
{
"name": "Center of Excellence on Environmental Health and Toxicology (EHT), OPS, MHESI, Bangkok, Thailand"
}
],
"family": "Rangkadilok",
"given": "Nuchanart",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Translational Research Unit, Chulabhorn Research Institute, Bangkok, Thailand"
}
],
"family": "Panomvana",
"given": "Duangchit",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Translational Research Unit, Chulabhorn Research Institute, Bangkok, Thailand"
}
],
"family": "Puranajoti",
"given": "Porranee",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Laboratory of Pharmacology, Chulabhorn Research Institute, Bangkok, Thailand"
},
{
"name": "Center of Excellence on Environmental Health and Toxicology (EHT), OPS, MHESI, Bangkok, Thailand"
}
],
"family": "Satayavivad",
"given": "Jutamaad",
"sequence": "additional"
}
],
"container-title": "Pharmaceutical Biology",
"container-title-short": "Pharmaceutical Biology",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"www.tandfonline.com"
]
},
"created": {
"date-parts": [
[
2025,
1,
6
]
],
"date-time": "2025-01-06T11:11:16Z",
"timestamp": 1736161876000
},
"deposited": {
"date-parts": [
[
2025,
1,
6
]
],
"date-time": "2025-01-06T11:11:21Z",
"timestamp": 1736161881000
},
"funder": [
{
"DOI": "10.13039/501100017170",
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100017170",
"id-type": "DOI"
}
],
"name": "Thailand Science Research and Innovation"
},
{
"DOI": "10.13039/501100007959",
"award": [
"2536713/43380",
"CRI-2567-01/3"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100007959",
"id-type": "DOI"
}
],
"name": "Chulabhorn Research Institute"
},
{
"name": "Thai Traditional Medical Knowledge Fund"
},
{
"name": "Department of Thai Traditional and Alternative Medicine"
},
{
"DOI": "10.13039/501100004397",
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100004397",
"id-type": "DOI"
}
],
"name": "Ministry of Public Health"
}
],
"indexed": {
"date-parts": [
[
2025,
1,
7
]
],
"date-time": "2025-01-07T05:17:05Z",
"timestamp": 1736227025227,
"version": "3.32.0"
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2025,
1,
6
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2025,
12,
31
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
1,
6
]
],
"date-time": "2025-01-06T00:00:00Z",
"timestamp": 1736121600000
}
}
],
"link": [
{
"URL": "https://www.tandfonline.com/doi/pdf/10.1080/13880209.2024.2444446",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "301",
"original-title": [],
"page": "42-52",
"prefix": "10.1080",
"published": {
"date-parts": [
[
2025,
1,
6
]
]
},
"published-online": {
"date-parts": [
[
2025,
1,
6
]
]
},
"published-print": {
"date-parts": [
[
2025,
12,
31
]
]
},
"publisher": "Informa UK Limited",
"reference": [
{
"DOI": "10.4103/jpbs.JPBS_175_19",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_2_1"
},
{
"article-title": "Effect of Andrographis paniculata treatment for patients with early-stage COVID-19 on the prevention of pneumonia: a retrospective cohort study",
"author": "Benjaponpitak A",
"first-page": "35",
"issue": "2",
"journal-title": "Arch. Intern. Med. Res. medRxiv",
"key": "e_1_3_5_3_1",
"unstructured": "Benjaponpitak A, Sawaengtham T, Thaneerat T, Wanaratna K, Chotsiri P, Rungsawang C, Bhubhanil S, Charoensuk S, Benjaponpitak S, Lapmanee S, et al. 2023. Effect of Andrographis paniculata treatment for patients with early-stage COVID-19 on the prevention of pneumonia: a retrospective cohort study. Arch. Intern. Med. Res. medRxiv. 6(2):35–43.",
"volume": "6",
"year": "2023"
},
{
"DOI": "10.1016/j.sciaf.2023.e01585",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_4_1"
},
{
"DOI": "10.3389/fphar.2022.950012",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_5_1"
},
{
"DOI": "10.1016/j.jtcme.2020.05.003",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_6_1"
},
{
"DOI": "10.1016/j.jtcme.2022.02.001",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_7_1"
},
{
"DOI": "10.1038/s41598-022-23189-7",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_8_1"
},
{
"article-title": "Andrographis paniculata (Burm. f.) Wall. ex Nees, andrographolide, and andrographolide analogues as SARS-CoV-2 antivirals? A rapid review",
"author": "Lim XY",
"first-page": "1",
"issue": "5",
"journal-title": "Nat Prod Commun",
"key": "e_1_3_5_9_1",
"unstructured": "Lim XY, Chan JSW, Tan TYC, Teh BP, Mohd Abd Razak MR, Mohamad S, Syed Mohamed AF. 2021. Andrographis paniculata (Burm. f.) Wall. ex Nees, andrographolide, and andrographolide analogues as SARS-CoV-2 antivirals? A rapid review. Nat Prod Commun. 16(5):1–15.",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1007/s13318-021-00736-7",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_10_1"
},
{
"DOI": "10.1038/s41586-022-05522-2",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_11_1"
},
{
"DOI": "10.1016/j.jep.2016.09.058",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_12_1"
},
{
"DOI": "10.1186/1749-8546-8-2",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_13_1"
},
{
"DOI": "10.1002/pca.3364",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_14_1"
},
{
"DOI": "10.3390/ph15081013",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_15_1"
},
{
"DOI": "10.4103/2229-3485.71856",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_16_1"
},
{
"DOI": "10.1021/acs.jnatprod.0c01324",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_17_1"
},
{
"DOI": "10.1016/j.ejps.2013.08.006",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_18_1"
},
{
"DOI": "10.1016/j.phymed.2023.155018",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_19_1"
},
{
"DOI": "10.1080/13880209.2024.2311201",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_20_1"
},
{
"DOI": "10.1038/s41598-023-28612-1",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_21_1"
},
{
"DOI": "10.3389/fphar.2023.1230401",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_22_1"
},
{
"DOI": "10.3389/fphar.2022.952660",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_23_1"
},
{
"DOI": "10.26502/aimr.0125",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_24_1"
},
{
"DOI": "10.1248/bpb.b22-00154",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_25_1"
}
],
"reference-count": 24,
"references-count": 24,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.tandfonline.com/doi/full/10.1080/13880209.2024.2444446"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Non-linear oral bioavailability and clinical pharmacokinetics of high-dose\n <i>Andrographis paniculata</i>\n ethanolic extract: relevant dosage implications for COVID-19 treatment",
"type": "journal-article",
"update-policy": "https://doi.org/10.1080/tandf_crossmark_01",
"volume": "63"
}