Effect of the P-Selectin Inhibitor Crizanlizumab on Survival Free of Organ Support in Patients Hospitalized for COVID-19: A Randomized Controlled Trial
et al., Circulation, doi:10.1161/CIRCULATIONAHA.123.065190, ACTIV-4a, NCT04505774, Aug 2023
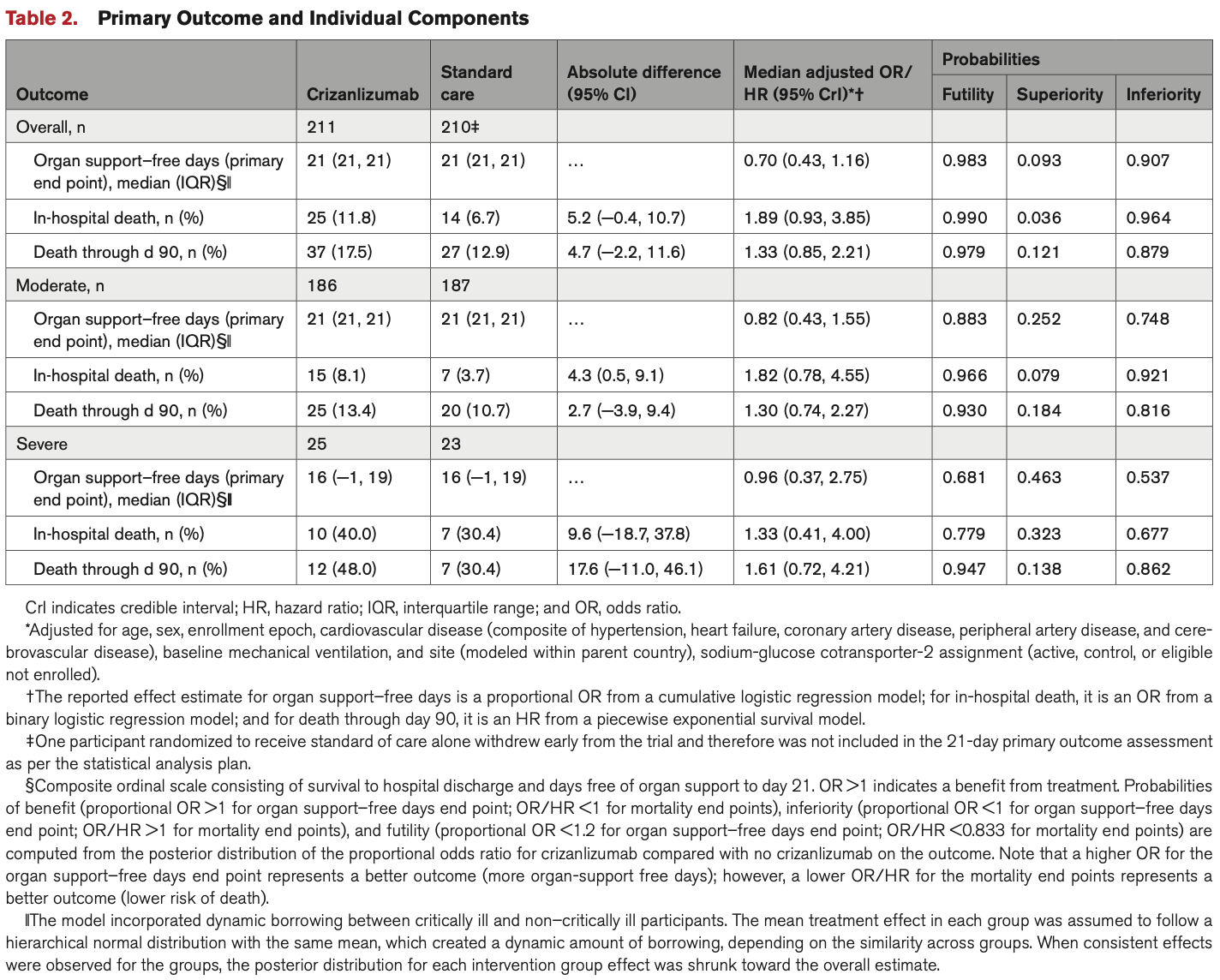
RCT 422 hospitalized COVID-19 patients showing no significant difference in mortality or organ support-free days with crizanlizumab, a P-selectin inhibitor. There was a trend towards increased mortality with crizanlizumab. The study was stopped early for futility.
|
risk of death, 33.0% higher, HR 1.33, p = 0.24, treatment 37 of 211 (17.5%), control 27 of 210 (12.9%), day 90.
|
|
risk of death, 78.4% higher, RR 1.78, p = 0.07, treatment 25 of 211 (11.8%), control 14 of 210 (6.7%), odds ratio converted to relative risk, in-hospital.
|
|
organ support-free days, 42.9% higher, OR 1.43, p = 0.16, treatment 211, control 210, inverted to make OR<1 favor treatment, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Solomon et al., 31 Aug 2023, Randomized Controlled Trial, multiple countries, peer-reviewed, mean age 68.0, 26 authors, study period 9 December, 2021 - 23 September, 2022, trial NCT04505774 (history) (ACTIV-4a).
Contact: ssolomon@bwh.harvard.edu.
Effect of the P-Selectin Inhibitor Crizanlizumab on Survival Free of Organ Support in Patients Hospitalized for COVID-19: A Randomized Controlled Trial
Circulation, doi:10.1161/circulationaha.123.065190
BACKGROUND: COVID-19 has been associated with endothelial injury, resultant microvascular inflammation and thrombosis. Activated endothelial cells release and express P-selectin and von Willebrand factor, both of which are elevated in severe COVID-19 and may be implicated in the disease pathophysiology. We hypothesized that crizanlizumab, a humanized monoclonal antibody to P-selectin, would reduce morbidity and death in patients hospitalized for COVID-19. METHODS: An international, adaptive, randomized controlled platform trial, funded by the National Heart, Lung, and Blood Institute, randomly assigned 422 patients hospitalized with COVID-19 with moderate or severe illness to receive either a single infusion of the P-selectin inhibitor crizanlizumab (at a dose of 5 mg/kg) plus standard of care or standard of care alone in an open-label 1:1 ratio. The primary outcome was organ support-free days, evaluated on an ordinal scale consisting of the number of days alive free of organ support through the first 21 days after trial entry.
RESULTS: The study was stopped for futility by the data safety monitoring committee. Among 421 randomized patients with known 21-day outcomes, 163 patients (77%) randomized to the crizanlizumab plus standard-of-care arm did not require any respiratory or cardiovascular organ support compared with 169 (80%) in the standard-of-care-alone arm. The adjusted odds ratio for the effect of crizanlizumab on organ support-free days was 0.70 (95% CI, 0.43-1.16), where an odds ratio >1 indicates treatment benefit, yielding a posterior probability of futility (odds ratio <1.2) of 98% and a posterior probability of inferiority (odds ratio <1.0) of 91%. Overall, there were 37 deaths (17.5%) in the crizanlizumab arm and 27 deaths (12.8%) in the standard-of-care arm (hazard ratio, 1.33 [95% CrI, 0.85-2.21]; [probability of hazard ratio>1] = 0.879).
CONCLUSIONS: Crizanlizumab, a P-selectin inhibitor, did not result in improvement in organ support-free days in patients hospitalized with COVID-19.
Sources of Funding The research was funded in part by the National Institutes of Health (NIH) agreement 1OT2HL156812 through the CONNECTS program. The views and conclusions contained in this document are those of the authors and should not be interpreted as representing the official policies, either expressed or implied, of NIH. Novartis provided the study drug and shipped it to study sites but had no role in the design or execution of the trial or in the analysis of the data.
Disclosures Dr Solomon has received research grants from Actelion, Alnylam, Amgen, AstraZeneca, Bellerophon, Bayer, BMS, Celladon, Cytokinetics, Eidos, Gilead, GSK, Ionis, Lilly, Mesoblast, MyoKardia, NIH/National Heart, Lung, and Blood Institute, Neurotronik, Novartis, NovoNordisk, Respicardia, Sanofi Pasteur, Theracos, and US2.AI. Dr Solomon has consulted for Abbott, Action, Akros, Alnylam, Amgen, Arena, AstraZeneca, Bayer, Boeringer-Ingelheim, BMS, Cardior, Cardurion, Corvia, Cytokinetics, Daiichi-Sankyo, GSK, Lilly, Merck, Myokardia, Novartis, Roche, Theracos, Quantum Genomics, Cardurion, Janssen, Cardiac Dimensions, Tenaya, Sanofi-Pasteur, Dinaqor, Tremeau, CellProThera, Moderna, American Regent, Sarepta, Lexicon, Anacardio, and Akros. Dr Lowenstein has received research grants from Novartis and NIH/National Heart, Lung, and Blood Institute (R01 HL134894, R33 HL14179, 1OT2HL156812, and R01 HL139553). Dr Peikert reports a research grant from the German Research Foundation. Dr Vardeny has received..
References
Ataga, Kutlar, Kanter, Liles, Cancado et al., Crizanlizumab for the prevention of pain crises in sickle cell disease, N Engl J Med, doi:10.1056/NEJMoa1611770
Barrett, Lee, Xia, Lin, Black et al., Platelet and vascular biomarkers associate with thrombosis and death in coronavirus disease, Circ Res, doi:10.1161/CIRCRESAHA.120.317803
Berger, Kornblith, Gong, Reynolds, Cushman et al., ACTIV-4a Investigators. Effect of P2Y12 inhibitors on survival free of organ support among noncritically ill hospitalized patients with COVID-19: a randomized clinical trial, JAMA, doi:10.1001/jama.2021.23605
Etulain, Martinod, Wong, Cifuni, Schattner et al., Pselectin promotes neutrophil extracellular trap formation in mice, Blood, doi:10.1182/blood-2015-01-624023
Goshua, Pine, Meizlish, Chang, Zhang et al., Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study, Lancet Haematol, doi:10.1016/S2352-3026(20)30216-7
Lawler, Goligher, Berger, Neal, Mcverry et al., Therapeutic anticoagulation with heparin in noncritically ill patients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2105911
Leucker, Osburn, Reventun, Smith, Claggett et al., Effect of crizanlizumab, a P-Selectin inhibitor, in COVID-19: a placebo-controlled, randomized trial, JACC Basic Transl Sci, doi:10.1016/j.jacbts.2021.09.013
Ley, Bullard, Arbonés, Bosse, Vestweber et al., Sequential contribution of L-and P-selectin to leukocyte rolling in vivo, J Exp Med, doi:10.1084/jem.181.2.669
Libby, Luscher, COVID-19 is, in the end, an endothelial disease, Eur Heart J, doi:10.1093/eurheartj/ehaa623
Lowenstein, Solomon, Severe COVID-19 is a microvascular disease, Circulation, doi:10.1161/CIRCULATIONAHA.120.050354
Matsukawa, Lukacs, Hogaboam, Knibbs, Bullard et al., Mice genetically lacking endothelial selectins are resistant to the lethality in septic peritonitis, Exp Mol Pathol, doi:10.1006/exmp.2001.2416
Mayadas, Johnson, Rayburn, Hynes, Wagner, Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice, Cell, doi:10.1016/0092-8674(93)80055-j
Mcever, Selectins: initiators of leucocyte adhesion and signalling at the vascular wall, Cardiovasc Res, doi:10.1093/cvr/cvv154
Md, Albert Einstein College of Medicine
Merten, Thiagarajan, P-selectin expression on platelets determines size and stability of platelet aggregates, Circulation, doi:10.1161/01.cir.102.16.1931
Myers Dd, Rectenwald, Bedard, Kaila, Shaw et al., Decreased venous thrombosis with an oral inhibitor of P selectin, J Vasc Surg, doi:10.1016/j.jvs.2005.04.045
Purdy, Obi, Myers, Wakefield, P-and E-selectin in venous thrombosis and non-venous pathologies, J Thromb Haemost, doi:10.1111/jth.15689
Robinson, Frenette, Rayburn, Cummiskey, Ullman-Culleré et al., Multiple, targeted deficiencies in selectins reveal a predominant role for P-selectin in leukocyte recruitment, Proc Natl Acad Sci U S A, doi:10.1073/pnas.96.20.11452
Zuo, Yalavarthi, Shi, Gockman, Zuo et al., Neutrophil extracellular traps in COVID-19, JCI Insight, doi:10.1172/jci.insight.138999
DOI record:
{
"DOI": "10.1161/circulationaha.123.065190",
"ISSN": [
"0009-7322",
"1524-4539"
],
"URL": "http://dx.doi.org/10.1161/CIRCULATIONAHA.123.065190",
"abstract": "<jats:sec>\n <jats:title>BACKGROUND:</jats:title>\n <jats:p>COVID-19 has been associated with endothelial injury, resultant microvascular inflammation and thrombosis. Activated endothelial cells release and express P-selectin and von Willebrand factor, both of which are elevated in severe COVID-19 and may be implicated in the disease pathophysiology. We hypothesized that crizanlizumab, a humanized monoclonal antibody to P-selectin, would reduce morbidity and death in patients hospitalized for COVID-19.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>METHODS:</jats:title>\n <jats:p>An international, adaptive, randomized controlled platform trial, funded by the National Heart, Lung, and Blood Institute, randomly assigned 422 patients hospitalized with COVID-19 with moderate or severe illness to receive either a single infusion of the P-selectin inhibitor crizanlizumab (at a dose of 5 mg/kg) plus standard of care or standard of care alone in an open-label 1:1 ratio. The primary outcome was organ support–free days, evaluated on an ordinal scale consisting of the number of days alive free of organ support through the first 21 days after trial entry.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>RESULTS:</jats:title>\n <jats:p>The study was stopped for futility by the data safety monitoring committee. Among 421 randomized patients with known 21-day outcomes, 163 patients (77%) randomized to the crizanlizumab plus standard-of-care arm did not require any respiratory or cardiovascular organ support compared with 169 (80%) in the standard-of-care–alone arm. The adjusted odds ratio for the effect of crizanlizumab on organ support–free days was 0.70 (95% CI, 0.43–1.16), where an odds ratio >1 indicates treatment benefit, yielding a posterior probability of futility (odds ratio <1.2) of 98% and a posterior probability of inferiority (odds ratio <1.0) of 91%. Overall, there were 37 deaths (17.5%) in the crizanlizumab arm and 27 deaths (12.8%) in the standard-of-care arm (hazard ratio, 1.33 [95% CrI, 0.85-2.21]; [probability of hazard ratio>1] = 0.879).</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>CONCLUSIONS:</jats:title>\n <jats:p>Crizanlizumab, a P-selectin inhibitor, did not result in improvement in organ support–free days in patients hospitalized with COVID-19.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>REGISTRATION:</jats:title>\n <jats:p>\n URL:\n <jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"uri\" xlink:href=\"https://www.clinicaltrials.gov\">https://www.clinicaltrials.gov</jats:ext-link>\n ; Unique identifier: NCT04505774.\n </jats:p>\n </jats:sec>",
"alternative-id": [
"10.1161/CIRCULATIONAHA.123.065190"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2023-04-16"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2023-05-12"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2023-06-25"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-3698-9597",
"affiliation": [
{
"name": "Cardiovascular Medicine Division, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA (S.D.S., A.S.B., A.P., A.B.)."
}
],
"authenticated-orcid": true,
"family": "Solomon",
"given": "Scott D.",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0003-0485-7514",
"affiliation": [
{
"name": "Johns Hopkins School of Medicine, Baltimore, MD (C.J.L.)."
}
],
"authenticated-orcid": true,
"family": "Lowenstein",
"given": "Charles J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Cardiovascular Medicine Division, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA (S.D.S., A.S.B., A.P., A.B.)."
},
{
"name": "Kaiser Permanente San Francisco Medical Center, CA (A.S.B.)."
}
],
"family": "Bhatt",
"given": "Ankeet S.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0947-5865",
"affiliation": [
{
"name": "Cardiovascular Medicine Division, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA (S.D.S., A.S.B., A.P., A.B.)."
}
],
"authenticated-orcid": true,
"family": "Peikert",
"given": "Alexander",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Minnesota and the Minneapolis VA Medical Center (O.V.)."
}
],
"family": "Vardeny",
"given": "Orly",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3750-9789",
"affiliation": [
{
"name": "Saint Luke’s Mid America Heart Institute, University of Missouri–Kansas City (M.N.K.)."
}
],
"authenticated-orcid": true,
"family": "Kosiborod",
"given": "Mikhail N.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8216-4647",
"affiliation": [
{
"name": "Cardiovascular Clinical Research Center, NYU Grossman School of Medicine, New York (J.S.B., H.R.R., S.M., J.S.H.)."
}
],
"authenticated-orcid": true,
"family": "Berger",
"given": "Jeffrey S.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0284-0655",
"affiliation": [
{
"name": "Cardiovascular Clinical Research Center, NYU Grossman School of Medicine, New York (J.S.B., H.R.R., S.M., J.S.H.)."
}
],
"authenticated-orcid": true,
"family": "Reynolds",
"given": "Harmony R.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Cardiovascular Clinical Research Center, NYU Grossman School of Medicine, New York (J.S.B., H.R.R., S.M., J.S.H.)."
}
],
"family": "Mavromichalis",
"given": "Stephanie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Cardiovascular Medicine Division, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA (S.D.S., A.S.B., A.P., A.B.)."
}
],
"family": "Barytol",
"given": "Anya",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8654-5014",
"affiliation": [
{
"name": "University of Pittsburgh, PA (A.D.A., J.F.L., M.W.G., M.D.N.)."
}
],
"authenticated-orcid": true,
"family": "Althouse",
"given": "Andrew D.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Pittsburgh, PA (A.D.A., J.F.L., M.W.G., M.D.N.)."
}
],
"family": "Luther",
"given": "James F.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "National Heart, Lung, and Blood Institute, Bethesda, MD (E.S.L., A.L.K.)."
}
],
"family": "Leifer",
"given": "Eric S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "National Heart, Lung, and Blood Institute, Bethesda, MD (E.S.L., A.L.K.)."
}
],
"family": "Kindzelski",
"given": "Andrei L.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7871-6143",
"affiliation": [
{
"name": "Larner College of Medicine, University of Vermont, Burlington (M.C.)."
}
],
"authenticated-orcid": true,
"family": "Cushman",
"given": "Mary",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7952-5384",
"affiliation": [
{
"name": "Albert Einstein College of Medicine, Bronx, NY (M.N.G.)."
}
],
"authenticated-orcid": true,
"family": "Gong",
"given": "Michelle N.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of California, San Francisco (L.Z.K.)."
}
],
"family": "Kornblith",
"given": "Lucy Z.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7344-8266",
"affiliation": [
{
"name": "University of Cincinnati Medical Center, OH (P.K.)."
}
],
"authenticated-orcid": true,
"family": "Khatri",
"given": "Pooja",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8480-4801",
"affiliation": [
{
"name": "University of Illinois, Chicago (K.S.K.)."
}
],
"authenticated-orcid": true,
"family": "Kim",
"given": "Keri S.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1171-0548",
"affiliation": [
{
"name": "Versity Blood Research Institute, Milwaukee, WI (L.B.K.)."
}
],
"authenticated-orcid": true,
"family": "Baumann Kreuziger",
"given": "Lisa",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2160-3287",
"affiliation": [
{
"name": "Duke University, Durham, NC (L.W.)."
}
],
"authenticated-orcid": true,
"family": "Wahid",
"given": "Lana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Socar Research SA, Nyon, Switzerland (B.-A.K.)."
}
],
"family": "Kirwan",
"given": "Bridget-Anne",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2280-3424",
"affiliation": [
{
"name": "University of Pittsburgh, PA (A.D.A., J.F.L., M.W.G., M.D.N.)."
}
],
"authenticated-orcid": true,
"family": "Geraci",
"given": "Mark W.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8931-6236",
"affiliation": [
{
"name": "University of Pittsburgh, PA (A.D.A., J.F.L., M.W.G., M.D.N.)."
}
],
"authenticated-orcid": true,
"family": "Neal",
"given": "Matthew D.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5889-5981",
"affiliation": [
{
"name": "Cardiovascular Clinical Research Center, NYU Grossman School of Medicine, New York (J.S.B., H.R.R., S.M., J.S.H.)."
}
],
"authenticated-orcid": true,
"family": "Hochman",
"given": "Judith S.",
"sequence": "additional"
},
{
"affiliation": [],
"name": "for the ACTIV4a Investigators",
"sequence": "additional"
}
],
"clinical-trial-number": [
{
"clinical-trial-number": "nct04505774",
"registry": "10.18810/clinical-trials-gov"
}
],
"container-title": "Circulation",
"container-title-short": "Circulation",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"www.ahajournals.org"
]
},
"created": {
"date-parts": [
[
2023,
6,
25
]
],
"date-time": "2023-06-25T14:45:10Z",
"timestamp": 1687704310000
},
"deposited": {
"date-parts": [
[
2024,
5,
13
]
],
"date-time": "2024-05-13T08:41:34Z",
"timestamp": 1715589694000
},
"funder": [
{
"DOI": "10.13039/100000050",
"award": [
"1OT2HL156812"
],
"doi-asserted-by": "crossref",
"id": [
{
"asserted-by": "crossref",
"id": "10.13039/100000050",
"id-type": "DOI"
}
],
"name": "National Heart Lung and Blood Institute"
}
],
"indexed": {
"date-parts": [
[
2024,
9,
11
]
],
"date-time": "2024-09-11T00:36:35Z",
"timestamp": 1726014995544
},
"is-referenced-by-count": 7,
"issue": "5",
"issued": {
"date-parts": [
[
2023,
8
]
]
},
"journal-issue": {
"issue": "5",
"published-print": {
"date-parts": [
[
2023,
8
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://www.ahajournals.org/doi/full/10.1161/CIRCULATIONAHA.123.065190",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "276",
"original-title": [],
"page": "381-390",
"prefix": "10.1161",
"published": {
"date-parts": [
[
2023,
8
]
]
},
"published-print": {
"date-parts": [
[
2023,
8
]
]
},
"publisher": "Ovid Technologies (Wolters Kluwer Health)",
"reference": [
{
"DOI": "10.1161/CIRCULATIONAHA.120.050354",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_2_2"
},
{
"DOI": "10.1093/eurheartj/ehaa623",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_3_2"
},
{
"DOI": "10.1016/S2352-3026(20)30216-7",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_4_2"
},
{
"DOI": "10.1161/CIRCRESAHA.120.317803",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_5_2"
},
{
"DOI": "10.1182/blood-2015-01-624023",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_6_2"
},
{
"DOI": "10.1172/jci.insight.138999",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_7_2"
},
{
"DOI": "10.1056/NEJMoa1611770",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_8_2"
},
{
"DOI": "10.1016/j.jacbts.2021.09.013",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_9_2"
},
{
"DOI": "10.1056/NEJMoa2105911",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_10_2"
},
{
"DOI": "10.1001/jama.2021.23605",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_11_2"
},
{
"DOI": "10.1093/cvr/cvv154",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_12_2"
},
{
"DOI": "10.1161/01.cir.102.16.1931",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_13_2"
},
{
"DOI": "10.1016/0092-8674(93)80055-j",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_14_2"
},
{
"DOI": "10.1006/exmp.2001.2416",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_15_2"
},
{
"DOI": "10.1016/j.jvs.2005.04.045",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_16_2"
},
{
"DOI": "10.1111/jth.15689",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_17_2"
},
{
"DOI": "10.1073/pnas.96.20.11452",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_18_2"
},
{
"DOI": "10.1084/jem.181.2.669",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_19_2"
}
],
"reference-count": 18,
"references-count": 18,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.123.065190"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Effect of the P-Selectin Inhibitor Crizanlizumab on Survival Free of Organ Support in Patients Hospitalized for COVID-19: A Randomized Controlled Trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1161/crossmarkpolicy",
"volume": "148"
}