Randomized clinical trial of nitazoxanide or sofosbuvir/daclatasvir for the prevention of SARS-CoV-2 infection
et al., Journal of Antimicrobial Chemotherapy, doi:10.1093/jac/dkac266, COVER, NCT04561063, Aug 2022
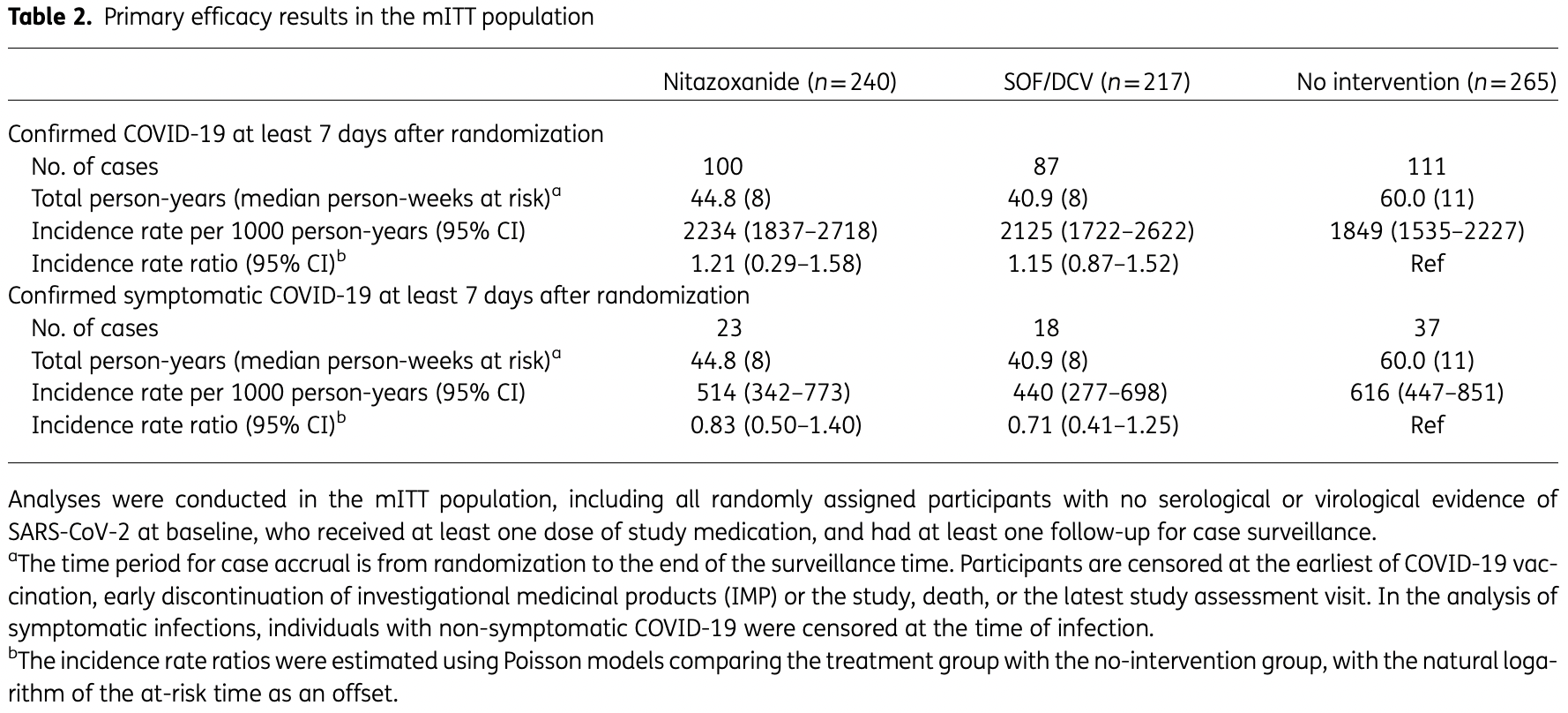
Prophylaxis RCT 828 high-risk participants in South Africa, showing no significant difference with nitazoxanide and sofosbuvir/daclatasvir treatment. FLU-PRO results were available for 74% of the nitazoxanide arm compared to 54% of the control arm.
|
risk of death, 65.6% lower, RR 0.34, p = 1.00, treatment 0 of 240 (0.0%), control 1 of 265 (0.4%), NNT 265, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of hospitalization, 79.2% lower, RR 0.21, p = 0.50, treatment 0 of 240 (0.0%), control 2 of 265 (0.8%), NNT 132, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of symptomatic case, 17.0% lower, RR 0.83, p = 0.49, treatment 23 of 240 (9.6%), control 37 of 265 (14.0%), incidence rate ratio
.
|
|
risk of case, 21.0% higher, RR 1.21, p = 0.67, treatment 23 of 240 (9.6%), control 37 of 265 (14.0%), incidence rate ratio
, primary outcome.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Sokhela et al., 12 Aug 2022, Randomized Controlled Trial, South Africa, peer-reviewed, median age 24.0, 11 authors, study period December 2020 - January 2022, trial NCT04561063 (history) (COVER).
Contact: ssokhela@ezintsha.org.
Randomized clinical trial of nitazoxanide or sofosbuvir/daclatasvir for the prevention of SARS-CoV-2 infection
Journal of Antimicrobial Chemotherapy, doi:10.1093/jac/dkac266
Background: The COVER trial evaluated whether nitazoxanide or sofosbuvir/daclatasvir could lower the risk of SARS-CoV-2 infection. Nitazoxanide was selected given its favourable pharmacokinetics and in vitro antiviral effects against SARS-CoV-2. Sofosbuvir/daclatasvir had shown favourable results in early clinical trials. Methods: In this clinical trial in Johannesburg, South Africa, healthcare workers and others at high risk of infection were randomized to 24 weeks of either nitazoxanide or sofosbuvir/daclatasvir as prevention, or standard prevention advice only. Participants were evaluated every 4 weeks for COVID-19 symptoms and had antibody and PCR testing. The primary endpoint was positive SARS-CoV-2 PCR and/or serology ≥7 days after randomization, regardless of symptoms. A Poisson regression model was used to estimate the incidence rate ratios of confirmed SARS-CoV-2 between each experimental arm and control.
References
Arshad, Pertinez, Box, Prioritization of anti-SARS-Cov-2 drug repurposing opportunities based on plasma and target site concentrations derived from their established human pharmacokinetics, Clin Pharmacol Ther, doi:10.1002/cpt.1909
Ayerdi, Puerta, Clavo, Preventive efficacy of tenofovir/emtricitabine against severe acute respiratory syndrome coronavirus 2 among pre-exposure prophylaxis users, Open Forum Infect Dis, doi:10.1093/ofid/ofaa455
Baden, Sahly, Essink, Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine, N Engl J Med, doi:10.1056/NEJMoa2035389
Baeten, Donnell, Ndase, Antiretroviral prophylaxis for HIV prevention in heterosexual men and women, N Engl J Med, doi:10.1056/NEJMoa1108524
Bbc News, New Omicron variant: Are low vaccination rates in South Africa a factor? 3
Blum, Cimerman, Hunter, Nitazoxanide superiority to placebo to treat moderate COVID-19 -a pilot prove of concept randomized double-blind clinical trial, EClinicalMedicine, doi:10.1016/j.eclinm.2021.100981
Cooper, Betsch, Sambala, Vaccine hesitancy -a potential threat to the achievements of vaccination programmes in Africa, Hum Vaccin Immunother, doi:10.1080/21645515.2018.1460987
Cooper, Van Rooyen, Wiysonge, COVID-19 vaccine hesitancy in South Africa: how can we maximize uptake of COVID-19 vaccines?, Expert Rev Vaccines, doi:10.1080/14760584.2021.1949291
Eslami, Mousaviasl, Radmanesh, The impact of sofosbuvir/ daclatasvir or ribavirin in patients with severe COVID-19, J Antimicrob Chemother, doi:10.1093/jac/dkaa331
Fowotade, Bamidele, Egbetola, An open label, adaptive, phase 1 trial of high-dose oral nitazoxanide in healthy volunteers: an antiviral candidate for SARS-CoV-2, Clin Pharmacol Ther, doi:10.1002/cpt.2463
Grant, Lama, Anderson, Preexposure chemoprophylaxis for HIV prevention in men who have sex with men, N Engl J Med, doi:10.1056/NEJMoa1011205
Griffiths, Fitzgerald, Jaki, AGILE: a seamless phase I/IIa platform for the rapid evaluation of candidates for COVID-19 treatment: an update to the structured summary of a study protocol for a randomised platform trial letter, Trials, doi:10.1186/s13063-021-05458-4
Hill, Mirchandani, Ellis, Ivermectin for the prevention of COVID-19: addressing potential bias and medical fraud, J Antimicrob Chemother, doi:10.1093/jac/dkac052
Jackson, Anderson, Rouphael, An mRNA vaccine against SARS-CoV-2-preliminary report, N Engl J Med, doi:10.1056/NEJMoa2022483
Kirby, Symonds, Kearney, Pharmacokinetic, pharmacodynamic, and drug-interaction profile of the hepatitis C virus NS5B polymerase inhibitor sofosbuvir, Clin Pharmacokinetics, doi:10.1007/s40262-015-0261-7
Lurie, Saville, Hatchett, Developing Covid-19 vaccines at pandemic speed, N Engl J Med, doi:10.1056/NEJMp2005630
Mendieta Zerón, Calderón, Coria, Nitazoxanide as an early treatment to reduce the intensity of COVID-19 outbreaks among health personnel, World Acad Sci J, doi:10.3892/wasj.2021.94
Messina, Nevola, Izzi, Efficacy and safety of the sofosbuvir/ velpatasvir combination for the treatment of patients with early mild to moderate COVID-19, Sci Rep, doi:10.1038/s41598-022-09741-5
Mobarak, Salasi, Hormati, Evaluation of the effect of sofosbuvir and daclatasvir in hospitalized COVID-19 patients: a randomized double-blind clinical trial (DISCOVER), J Antimicrob Chemother, doi:10.1093/jac/dkab433
Nguyen, Drew, Graham, Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study, Lancet Public Health, doi:10.1016/S2468-2667(20)30164-X
Pfizer, Pfizer and BioNTech Conclude Phase 3 Study of COVID-19
Powers, Guerrero, Leidy, Development of the Flu-PRO: a patient-reported outcome (PRO) instrument to evaluate symptoms of influenza, BMC Infect Dis, doi:10.1186/s12879-015-1330-0
Rajoli, Pertinez, Arshad, Dose prediction for repurposing nitazoxanide in SARS-CoV-2 treatment or chemoprophylaxis, Br J Clin Pharmacol, doi:10.1111/bcp.14619
Ritchie, Mathieu, Rodés-Guirao, Coronavirus Pandemic (COVID-19
Saag, Gandhi, Hoy, Antiretroviral drugs for treatment and prevention of HIV infection in adults, JAMA, doi:10.1001/jama.2020.17025
Sacramento, Fintelman-Rodrigues, Temerozo, In vitro antiviral activity of the anti-HCV drugs daclatasvir and sofosbuvir against SARS-CoV-2, the aetiological agent of COVID-19, J Antimicrob Chemother, doi:10.1093/jac/dkab072
Schmidt, Why Is Omicron So Contagious? Scientific American
Simmons, Wentzel, Mobarak, Sofosbuvir/daclatasvir regimens for the treatment of COVID-19: an individual patient data meta-analysis, J Antimicrob Chemother, doi:10.1093/jac/dkaa418
Subarrao, COVID-19 vaccines: time to talk about the uncertainties, Nature, doi:10.1038/d41586-020-02944-8
Us Fda, Why you should not use ivermectin to treat or prevent COVID-19
Who, Coronavirus disease (COVID-19): hydroxychloroquine
Who, Drugs to prevent COVID-19: a WHO living guideline
Who, WHO updates its treatment guidelines to include molnupiravir
Worldometer, Coronavirus Statistics: South Africa
Zein, Sulistiyana, Raffaello, Sofosbuvir with daclatasvir and the outcomes of patients with COVID-19: a systematic review and meta-analysis with GRADE assessment, Postgrad Med J, doi:10.1136/postgradmedj-2021-140287
DOI record:
{
"DOI": "10.1093/jac/dkac266",
"ISSN": [
"0305-7453",
"1460-2091"
],
"URL": "http://dx.doi.org/10.1093/jac/dkac266",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>The COVER trial evaluated whether nitazoxanide or sofosbuvir/daclatasvir could lower the risk of SARS-CoV-2 infection. Nitazoxanide was selected given its favourable pharmacokinetics and in vitro antiviral effects against SARS-CoV-2. Sofosbuvir/daclatasvir had shown favourable results in early clinical trials.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>In this clinical trial in Johannesburg, South Africa, healthcare workers and others at high risk of infection were randomized to 24 weeks of either nitazoxanide or sofosbuvir/daclatasvir as prevention, or standard prevention advice only. Participants were evaluated every 4 weeks for COVID-19 symptoms and had antibody and PCR testing. The primary endpoint was positive SARS-CoV-2 PCR and/or serology ≥7 days after randomization, regardless of symptoms. A Poisson regression model was used to estimate the incidence rate ratios of confirmed SARS-CoV-2 between each experimental arm and control.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>Between December 2020 and January 2022, 828 participants were enrolled. COVID-19 infections were confirmed in 100 participants on nitazoxanide (2234 per 1000 person-years; 95% CI 1837–2718), 87 on sofosbuvir/daclatasvir (2125 per 1000 person-years; 95% CI 1722–2622) and 111 in the control arm (1849 per 1000 person-years; 95% CI 1535–2227). There were no significant differences in the primary endpoint between the treatment arms, and the results met the criteria for futility. In the safety analysis, the frequency of grade 3 or 4 adverse events was low and similar across arms.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>In this randomized trial, nitazoxanide and sofosbuvir/daclatasvir had no significant preventative effect on infection with SARS-CoV-2 among healthcare workers and others at high risk of infection.</jats:p>\n </jats:sec>",
"author": [
{
"ORCID": "http://orcid.org/0000-0002-2707-1533",
"affiliation": [
{
"name": "Ezintsha, Faculty of Health Sciences, University of the Witwatersrand , Johannesburg , South Africa"
}
],
"authenticated-orcid": false,
"family": "Sokhela",
"given": "Simiso",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Ezintsha, Faculty of Health Sciences, University of the Witwatersrand , Johannesburg , South Africa"
}
],
"family": "Bosch",
"given": "Bronwyn",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pharmacology and Therapeutics, University of Liverpool , Liverpool , UK"
}
],
"family": "Hill",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "LSE Health, London School of Economics & Political Science , London , UK"
}
],
"family": "Simmons",
"given": "Bryony",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Ezintsha, Faculty of Health Sciences, University of the Witwatersrand , Johannesburg , South Africa"
}
],
"family": "Woods",
"given": "Joana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "HJ-Clinical Trial Consultancy , George , South Africa"
}
],
"family": "Johnstone",
"given": "Hilary",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Ezintsha, Faculty of Health Sciences, University of the Witwatersrand , Johannesburg , South Africa"
}
],
"family": "Akpomiemie",
"given": "Godspower",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Imperial College London, School of Public Health , London , UK"
}
],
"family": "Ellis",
"given": "Leah",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9819-7651",
"affiliation": [
{
"name": "Department of Pharmacology and Therapeutics, University of Liverpool , Liverpool , UK"
}
],
"authenticated-orcid": false,
"family": "Owen",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Unitaid, Global Health Campus , Chem. du Pommier 40, 1218 Le Grand-Saconnex , Switzerland"
}
],
"family": "Casas",
"given": "Carmen Perez",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4157-732X",
"affiliation": [
{
"name": "Ezintsha, Faculty of Health Sciences, University of the Witwatersrand , Johannesburg , South Africa"
}
],
"authenticated-orcid": false,
"family": "Venter",
"given": "Willem Daniel Francois",
"sequence": "additional"
}
],
"container-title": "Journal of Antimicrobial Chemotherapy",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
8,
12
]
],
"date-time": "2022-08-12T03:49:08Z",
"timestamp": 1660276148000
},
"deposited": {
"date-parts": [
[
2022,
8,
12
]
],
"date-time": "2022-08-12T03:49:38Z",
"timestamp": 1660276178000
},
"funder": [
{
"award": [
"2016-07-Wits"
],
"name": "RHI"
}
],
"indexed": {
"date-parts": [
[
2022,
8,
13
]
],
"date-time": "2022-08-13T04:28:59Z",
"timestamp": 1660364939996
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
8,
12
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
8,
12
]
],
"date-time": "2022-08-12T00:00:00Z",
"timestamp": 1660262400000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/jac/advance-article-pdf/doi/10.1093/jac/dkac266/45349402/dkac266.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/jac/advance-article-pdf/doi/10.1093/jac/dkac266/45349402/dkac266.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2022,
8,
12
]
]
},
"published-online": {
"date-parts": [
[
2022,
8,
12
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference": [
{
"DOI": "10.1038/d41586-020-02944-8",
"article-title": "COVID-19 vaccines: time to talk about the uncertainties",
"author": "Subarrao",
"doi-asserted-by": "crossref",
"first-page": "475",
"journal-title": "Nature",
"key": "2022081203470166300_dkac266-B1",
"volume": "586",
"year": "2020"
},
{
"DOI": "10.1056/NEJMp2005630",
"article-title": "Developing Covid-19 vaccines at pandemic speed",
"author": "Lurie",
"doi-asserted-by": "crossref",
"first-page": "1969",
"journal-title": "N Engl J Med",
"key": "2022081203470166300_dkac266-B2",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.17025",
"article-title": "Antiretroviral drugs for treatment and prevention of HIV infection in adults",
"author": "Saag",
"doi-asserted-by": "crossref",
"first-page": "1651",
"journal-title": "JAMA",
"key": "2022081203470166300_dkac266-B3",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa1011205",
"article-title": "Preexposure chemoprophylaxis for HIV prevention in men who have sex with men",
"author": "Grant",
"doi-asserted-by": "crossref",
"first-page": "2587",
"journal-title": "N Engl J Med",
"key": "2022081203470166300_dkac266-B4",
"volume": "363",
"year": "2010"
},
{
"DOI": "10.1056/NEJMoa1108524",
"article-title": "Antiretroviral prophylaxis for HIV prevention in heterosexual men and women",
"author": "Baeten",
"doi-asserted-by": "crossref",
"first-page": "399",
"journal-title": "N Engl J Med",
"key": "2022081203470166300_dkac266-B5",
"volume": "367",
"year": "2012"
},
{
"author": "WHO",
"key": "2022081203470166300_dkac266-B6",
"year": "2021"
},
{
"author": "WHO",
"key": "2022081203470166300_dkac266-B7",
"year": "2021"
},
{
"DOI": "10.1093/jac/dkac052",
"article-title": "Ivermectin for the prevention of COVID-19: addressing potential bias and medical fraud",
"author": "Hill",
"doi-asserted-by": "crossref",
"first-page": "1413",
"journal-title": "J Antimicrob Chemother",
"key": "2022081203470166300_dkac266-B8",
"volume": "77",
"year": "2022"
},
{
"author": "US FDA",
"key": "2022081203470166300_dkac266-B9",
"year": "2021"
},
{
"DOI": "10.1093/ofid/ofaa455",
"article-title": "Preventive efficacy of tenofovir/emtricitabine against severe acute respiratory syndrome coronavirus 2 among pre-exposure prophylaxis users",
"author": "Ayerdi",
"doi-asserted-by": "crossref",
"first-page": "ofaa455",
"journal-title": "Open Forum Infect Dis",
"key": "2022081203470166300_dkac266-B10",
"volume": "7",
"year": "2020"
},
{
"author": "WHO",
"key": "2022081203470166300_dkac266-B11",
"year": "2022"
},
{
"author": "NIH",
"key": "2022081203470166300_dkac266-B12",
"year": "2022"
},
{
"author": "NIH",
"key": "2022081203470166300_dkac266-B13",
"year": "2022"
},
{
"author": "Pfizer",
"key": "2022081203470166300_dkac266-B14",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2035389",
"article-title": "Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine",
"author": "Baden",
"doi-asserted-by": "crossref",
"first-page": "403",
"journal-title": "N Engl J Med",
"key": "2022081203470166300_dkac266-B15",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2022483",
"article-title": "An mRNA vaccine against SARS-CoV-2—preliminary report",
"author": "Jackson",
"doi-asserted-by": "crossref",
"first-page": "1920",
"journal-title": "N Engl J Med",
"key": "2022081203470166300_dkac266-B16",
"volume": "383",
"year": "2020"
},
{
"author": "Worldometer",
"key": "2022081203470166300_dkac266-B17",
"year": "2022"
},
{
"DOI": "10.1016/S2468-2667(20)30164-X",
"article-title": "Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study",
"author": "Nguyen",
"doi-asserted-by": "crossref",
"first-page": "e475",
"journal-title": "Lancet Public Health",
"key": "2022081203470166300_dkac266-B18",
"volume": "5",
"year": "2020"
},
{
"author": "SA COVID-19 Online Resource & News Portal",
"key": "2022081203470166300_dkac266-B19",
"year": "2022"
},
{
"DOI": "10.1002/cpt.1909",
"article-title": "Prioritization of anti-SARS-Cov-2 drug repurposing opportunities based on plasma and target site concentrations derived from their established human pharmacokinetics",
"author": "Arshad",
"doi-asserted-by": "crossref",
"first-page": "775",
"journal-title": "Clin Pharmacol Ther",
"key": "2022081203470166300_dkac266-B20",
"volume": "108",
"year": "2020"
},
{
"DOI": "10.1111/bcp.14619",
"article-title": "Dose prediction for repurposing nitazoxanide in SARS-CoV-2 treatment or chemoprophylaxis",
"author": "Rajoli",
"doi-asserted-by": "crossref",
"first-page": "2078",
"journal-title": "Br J Clin Pharmacol",
"key": "2022081203470166300_dkac266-B21",
"volume": "87",
"year": "2020"
},
{
"DOI": "10.1186/s13063-021-05458-4",
"article-title": "AGILE: a seamless phase I/IIa platform for the rapid evaluation of candidates for COVID-19 treatment: an update to the structured summary of a study protocol for a randomised platform trial letter",
"author": "Griffiths",
"doi-asserted-by": "crossref",
"first-page": "487",
"journal-title": "Trials",
"key": "2022081203470166300_dkac266-B22",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1093/jac/dkaa418",
"article-title": "Sofosbuvir/daclatasvir regimens for the treatment of COVID-19: an individual patient data meta-analysis",
"author": "Simmons",
"doi-asserted-by": "crossref",
"first-page": "286",
"journal-title": "J Antimicrob Chemother",
"key": "2022081203470166300_dkac266-B23",
"volume": "76",
"year": "2020"
},
{
"DOI": "10.1093/jac/dkab433",
"article-title": "Evaluation of the effect of sofosbuvir and daclatasvir in hospitalized COVID-19 patients: a randomized double-blind clinical trial (DISCOVER)",
"author": "Mobarak",
"doi-asserted-by": "crossref",
"first-page": "758",
"journal-title": "J Antimicrob Chemother",
"key": "2022081203470166300_dkac266-B24",
"volume": "77",
"year": "2021"
},
{
"DOI": "10.1007/s40262-015-0261-7",
"article-title": "Pharmacokinetic, pharmacodynamic, and drug-interaction profile of the hepatitis C virus NS5B polymerase inhibitor sofosbuvir",
"author": "Kirby",
"doi-asserted-by": "crossref",
"first-page": "677",
"journal-title": "Clin Pharmacokinetics",
"key": "2022081203470166300_dkac266-B25",
"volume": "54",
"year": "2015"
},
{
"DOI": "10.1093/jac/dkab072",
"article-title": "In vitro antiviral activity of the anti-HCV drugs daclatasvir and sofosbuvir against SARS-CoV-2, the aetiological agent of COVID-19",
"author": "Sacramento",
"doi-asserted-by": "crossref",
"first-page": "1874",
"journal-title": "J Antimicrob Chemother",
"key": "2022081203470166300_dkac266-B26",
"volume": "76",
"year": "2021"
},
{
"author": "NIH ClinRegs",
"key": "2022081203470166300_dkac266-B27",
"year": "2021"
},
{
"author": "WHO",
"key": "2022081203470166300_dkac266-B28",
"year": "2021"
},
{
"DOI": "10.1186/s12879-015-1330-0",
"article-title": "Development of the Flu-PRO: a patient-reported outcome (PRO) instrument to evaluate symptoms of influenza",
"author": "Powers",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "BMC Infect Dis",
"key": "2022081203470166300_dkac266-B29",
"volume": "16",
"year": "2016"
},
{
"DOI": "10.1136/postgradmedj-2021-140287",
"article-title": "Sofosbuvir with daclatasvir and the outcomes of patients with COVID-19: a systematic review and meta-analysis with GRADE assessment",
"author": "Zein",
"doi-asserted-by": "crossref",
"first-page": "509",
"journal-title": "Postgrad Med J",
"key": "2022081203470166300_dkac266-B30",
"volume": "98",
"year": "2022"
},
{
"DOI": "10.1038/s41598-022-09741-5",
"article-title": "Efficacy and safety of the sofosbuvir/velpatasvir combination for the treatment of patients with early mild to moderate COVID-19",
"author": "Messina",
"doi-asserted-by": "crossref",
"first-page": "5771",
"journal-title": "Sci Rep",
"key": "2022081203470166300_dkac266-B31",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1093/jac/dkaa331",
"article-title": "The impact of sofosbuvir/daclatasvir or ribavirin in patients with severe COVID-19",
"author": "Eslami",
"doi-asserted-by": "crossref",
"first-page": "3366",
"journal-title": "J Antimicrob Chemother",
"key": "2022081203470166300_dkac266-B32",
"volume": "75",
"year": "2020"
},
{
"DOI": "10.1016/j.eclinm.2021.100981",
"article-title": "Nitazoxanide superiority to placebo to treat moderate COVID-19 – a pilot prove of concept randomized double-blind clinical trial",
"author": "Blum",
"doi-asserted-by": "crossref",
"first-page": "100981",
"journal-title": "EClinicalMedicine",
"key": "2022081203470166300_dkac266-B33",
"volume": "37",
"year": "2021"
},
{
"DOI": "10.3892/wasj.2021.94",
"article-title": "Nitazoxanide as an early treatment to reduce the intensity of COVID-19 outbreaks among health personnel",
"author": "Mendieta Zerón",
"doi-asserted-by": "crossref",
"first-page": "23",
"journal-title": "World Acad Sci J",
"key": "2022081203470166300_dkac266-B34",
"volume": "3",
"year": "2021"
},
{
"article-title": "Efficacy and safety of nitazoxanide combined with ritonavir-boosted atazanavir for the treatment of mild to moderate COVID-19",
"author": "Fowotade",
"journal-title": "medRxiv",
"key": "2022081203470166300_dkac266-B35",
"year": "2022"
},
{
"DOI": "10.1002/cpt.2463",
"article-title": "An open label, adaptive, phase 1 trial of high-dose oral nitazoxanide in healthy volunteers: an antiviral candidate for SARS-CoV-2",
"author": "Walker",
"doi-asserted-by": "crossref",
"first-page": "585",
"journal-title": "Clin Pharmacol Ther",
"key": "2022081203470166300_dkac266-B36",
"volume": "111",
"year": "2021"
},
{
"author": "Schmidt",
"key": "2022081203470166300_dkac266-B37"
},
{
"DOI": "10.1080/21645515.2018.1460987",
"article-title": "Vaccine hesitancy – a potential threat to the achievements of vaccination programmes in Africa",
"author": "Cooper",
"doi-asserted-by": "crossref",
"first-page": "2355",
"journal-title": "Hum Vaccin Immunother",
"key": "2022081203470166300_dkac266-B38",
"volume": "14",
"year": "2018"
},
{
"DOI": "10.1080/14760584.2021.1949291",
"article-title": "COVID-19 vaccine hesitancy in South Africa: how can we maximize uptake of COVID-19 vaccines?",
"author": "Cooper",
"doi-asserted-by": "crossref",
"first-page": "921",
"journal-title": "Expert Rev Vaccines",
"key": "2022081203470166300_dkac266-B39",
"volume": "20",
"year": "2021"
},
{
"author": "BBC News",
"key": "2022081203470166300_dkac266-B40"
},
{
"author": "Ritchie",
"key": "2022081203470166300_dkac266-B41",
"year": "2022"
},
{
"author": "WHO",
"key": "2022081203470166300_dkac266-B42",
"year": "2021"
},
{
"author": "ClinicalTrials.gov",
"key": "2022081203470166300_dkac266-B43",
"year": "2022"
}
],
"reference-count": 43,
"references-count": 43,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/jac/advance-article/doi/10.1093/jac/dkac266/6661458"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Pharmacology (medical)",
"Pharmacology",
"Microbiology (medical)"
],
"subtitle": [],
"title": "Randomized clinical trial of nitazoxanide or sofosbuvir/daclatasvir for the prevention of SARS-CoV-2 infection",
"type": "journal-article"
}