An open label, adaptive, phase 1 trial of high-dose oral nitazoxanide in healthy volunteers: an antiviral candidate for SARS-CoV-2
et al., Clinical Pharmacology & Therapeutics, doi:10.1002/cpt.2463, NCT04746183, Sep 2021 (preprint)
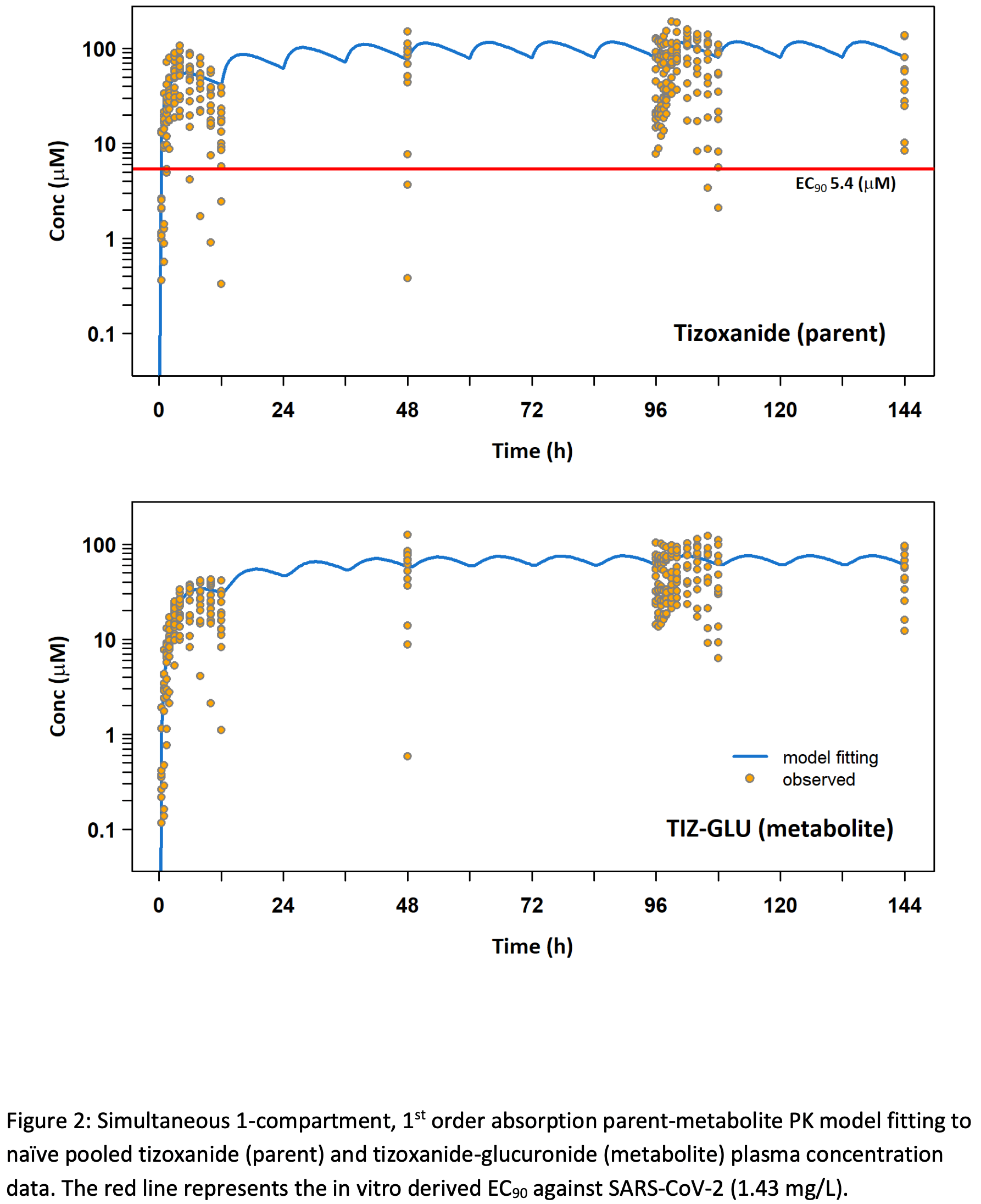
Phase I trial of high dose nitazoxanide, 1500mg twice daily, with 14 participants. Treatment was safe and well tolerated. PBPK predictions were confirmed on day 1 but with underprediction at day 5. Median Cmin was above the in vitro target concentration after the first dose and maintained throughout. NCT04746183 (history).
Walker et al., 11 Sep 2021, peer-reviewed, 33 authors, trial NCT04746183 (history).
An Open Label, Adaptive, Phase 1 Trial of High‐Dose Oral Nitazoxanide in Healthy Volunteers: An Antiviral Candidate for SARS‐CoV‐2
Clinical Pharmacology & Therapeutics, doi:10.1002/cpt.2463
This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record.
References
Arshad, Prioritization of Anti-SARS-Cov-2 Drug Repurposing Opportunities Based on Plasma and Target Site Concentrations Derived from their Established Human Pharmacokinetics, Clin Pharmacol Ther
Blum, Nitazoxanide superiority to placebo to treat moderate COVID-19 -A Pilot prove of concept randomized double-blind clinical trial, EClinicalMedicine
Bobrowski, Discovery of Synergistic and Antagonistic Drug Combinations against SARS-CoV-2 In Vitro
Boffito, Toward Consensus on Correct Interpretation of Protein Binding in Plasma and Other Biological Matrices for COVID-19 Therapeutic Development, Clin Pharmacol Ther
Borchers, pracma: Practical Numerical Math Functions. R package version 2
Braga, Drugs that inhibit TMEM16 proteins block SARS-CoV-2 spike-induced syncytia, Nature
Cao, Forrest, Zhang, A screen of the NIH Clinical Collection small molecule library identifies potential anti-coronavirus drugs, Antiviral Res
Choi, Arya, Reynolds, Clinical Pharmacology-Informed Development of COVID-19 Therapeutics: Regulatory Experience, Clin Pharmacol Ther
Dang, Nitazoxanide Inhibits Human Norovirus Replication and Synergizes with Ribavirin by Activation of Cellular Antiviral Response, Antimicrob Agents Chemother
Dang, Opposing Effects of Nitazoxanide on Murine and Human Norovirus, J Infect Dis
Griffiths, ACCORD: A Randomized, Multicentre, Seamless, Adaptive Phase I/II Platform Study to Determine the Optimal Dose, Safety and Efficacy of Multiple Candidate Agents for the Treatment of COVID-19: A structured summary of a study protocol for a randomised platform trial, Trials
Haffizulla, Effect of nitazoxanide in adults and adolescents with acute uncomplicated influenza: a double-blind, randomised, placebo-controlled, phase 2b/3 trial, Lancet Infect Dis
Hiscox, Shutting the gate before the horse has bolted: is it time for a conversation about SARS-CoV-2 and antiviral drug resistance?, J Antimicrob Chemother
Hoffmann, Chloroquine does not inhibit infection of human lung cells with SARS-CoV-2, Nature
Keiser, The drugs we ahve and the drugs we need against major helminth infections
Korba, Nitazoxanide, tizoxanide and other thiazolides are potent inhibitors of hepatitis B virus and hepatitis C virus replication, Antiviral Res
Korba, Potential for hepatitis C virus resistance to nitazoxanide or tizoxanide, Antimicrob Agents Chemother
Koszalka, Tilmanis, Hurt, Influenza antivirals currently in late-phase clinical trial, Influenza Other Respir Viruses
Maurer, Nonspecific Binding Considerations in the Rational Design and Development of Small Molecule COVID-19 Therapeutics, Clin Pharmacol Ther
Miner, Drug Repurposing: The Anthelmintics Niclosamide and Nitazoxanide Are Potent TMEM16A Antagonists That Fully Bronchodilate Airways, Front Pharmacol
Portal, Nitazoxanide, None
Rajoli, Dose prediction for repurposing nitazoxanide in SARS-CoV-2 treatment or chemoprophylaxis, Br J Clin Pharmacol
Rajoli, Dose prediction for repurposing nitazoxanide in SARS-CoV-2 treatment or chemoprophylaxis, British Journal of Clinical Pharmacology
Rannard, Mcdonald, Owen, Chasing COVID-19 chemotherapeutics without putting the cart before the horse, Br J Clin Pharmacol
Riccio, Rossi, Piacentini, Rossignol, Santoro, Impairment of SARS-CoV-2 spike glycoprotein maturation and fusion activity by the broad-spectrum anti-infective drug nitazoxanide
Rosenke, Hydroxychloroquine Proves Ineffective in Hamsters and Macaques Infected with SARS-CoV-2
Rossignol, El-Gohary, Nitazoxanide in the treatment of viral gastroenteritis: a randomized double-blind placebo-controlled clinical trial, Aliment Pharmacol Ther
Rossignol, Nitazoxanide: a first-in-class broad-spectrum antiviral agent, Antiviral Res
Rossignol, Oaks, Bostick, Vora, Fulgencio et al., Early treatment with nitazoxanide prevents worsening of mild and moderate COVID-19 and subsequent hospitalization
Rossignol, Thiazolides, a new class of anti-influenza molecules targeting viral hemagglutinin at the post-translational level, J Biol Chem
Rossignol, Thiazolides: a new class of antiviral drugs, Expert Opin Drug Metab Toxicol
Savic, Population pharmacokinetics of rifapentine and desacetyl rifapentine in healthy volunteers: nonlinearities in clearance and bioavailability, Antimicrob Agents Chemother
Stockis, Nitazoxanide pharmacokinetics and tolerability in man during 7 days dosing with 0.5 g and 1 g b.i.d, Int J Clin Pharmacol Ther
Team, R: A language and environment for statistical computing
Thorlund, A real-time dashboard of clinical trials for COVID-19, Lancet Digit Health
Trabattoni, Thiazolides Elicit Anti-Viral Innate Immunity and Reduce HIV Replication, Sci Rep
Wages, Conaway, O'quigley, Continual reassessment method for partial ordering, Biometrics
Wang, Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res
DOI record:
{
"DOI": "10.1002/cpt.2463",
"ISSN": [
"0009-9236",
"1532-6535"
],
"URL": "http://dx.doi.org/10.1002/cpt.2463",
"abstract": "<jats:p>Repurposing approved drugs may rapidly establish effective interventions during a public health crisis. This has yielded immunomodulatory treatments for severe coronavirus disease 2019 (COVID‐19), but repurposed antivirals have not been successful to date because of redundancy of the target <jats:italic>in vivo</jats:italic> or suboptimal exposures at studied doses. Nitazoxanide is a US Food and Drug Administration (FDA) approved antiparasitic medicine, that physiologically‐based pharmacokinetic (PBPK) modeling has indicated may provide antiviral concentrations across the dosing interval, when repurposed at higher than approved doses. Within the AGILE trial platform (NCT04746183) an open label, adaptive, phase I trial in healthy adult participants was undertaken with high‐dose nitazoxanide. Participants received 1,500 mg nitazoxanide orally twice‐daily with food for 7 days. Primary outcomes were safety, tolerability, optimum dose, and schedule. Intensive pharmacokinetic (PK) sampling was undertaken day 1 and 5 with minimum concentration (C<jats:sub>min</jats:sub>) sampling on days 3 and 7. Fourteen healthy participants were enrolled between February 18 and May 11, 2021. All 14 doses were completed by 10 of 14 participants. Nitazoxanide was safe and with no significant adverse events. Moderate gastrointestinal disturbance (loose stools or diarrhea) occurred in 8 participants (57.1%), with urine and sclera discoloration in 12 (85.7%) and 9 (64.3%) participants, respectively, without clinically significant bilirubin elevation. This was self‐limiting and resolved upon drug discontinuation. PBPK predictions were confirmed on day 1 but with underprediction at day 5. Median C<jats:sub>min</jats:sub> was above the <jats:italic>in vitro</jats:italic> target concentration on the first dose and maintained throughout. Nitazoxanide administered at 1,500 mg b.i.d. with food was safe with acceptable tolerability a phase Ib/IIa study is now being initiated in patients with COVID‐19.</jats:p>",
"alternative-id": [
"10.1002/cpt.2463"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2021-08-09"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2021-10-16"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2021-11-13"
}
],
"author": [
{
"affiliation": [
{
"name": "University of Liverpool Liverpool UK"
},
{
"name": "Liverpool University Hospitals NHS Foundation Trust Liverpool UK"
}
],
"family": "Walker",
"given": "Lauren E.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Liverpool University Hospitals NHS Foundation Trust Liverpool UK"
}
],
"family": "FitzGerald",
"given": "Richard",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Southampton Clinical Trials Unit University of Southampton Southampton UK"
}
],
"family": "Saunders",
"given": "Geoffrey",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Liverpool University Hospitals NHS Foundation Trust Liverpool UK"
}
],
"family": "Lyon",
"given": "Rebecca",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2304-6434",
"affiliation": [
{
"name": "University of Liverpool Liverpool UK"
},
{
"name": "Liverpool University Hospitals NHS Foundation Trust Liverpool UK"
}
],
"authenticated-orcid": false,
"family": "Fisher",
"given": "Michael",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6362-0501",
"affiliation": [
{
"name": "Southampton Clinical Trials Unit University of Southampton Southampton UK"
}
],
"authenticated-orcid": false,
"family": "Martin",
"given": "Karen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Southampton Clinical Trials Unit University of Southampton Southampton UK"
}
],
"family": "Eberhart",
"given": "Izabela",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Liverpool University Hospitals NHS Foundation Trust Liverpool UK"
}
],
"family": "Woods",
"given": "Christie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Southampton Clinical Trials Unit University of Southampton Southampton UK"
}
],
"family": "Ewings",
"given": "Sean",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Liverpool University Hospitals NHS Foundation Trust Liverpool UK"
}
],
"family": "Hale",
"given": "Colin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Liverpool Liverpool UK"
}
],
"family": "Rajoli",
"given": "Rajith K. R.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Liverpool Liverpool UK"
}
],
"family": "Else",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Liverpool Liverpool UK"
}
],
"family": "Dilly‐Penchala",
"given": "Sujan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Liverpool Liverpool UK"
}
],
"family": "Amara",
"given": "Alieu",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Liverpool School of Tropical Medicine Liverpool UK"
}
],
"family": "Lalloo",
"given": "David G.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Liverpool School of Tropical Medicine Liverpool UK"
}
],
"family": "Jacobs",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Liverpool Liverpool UK"
}
],
"family": "Pertinez",
"given": "Henry",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Southampton Clinical Trials Unit University of Southampton Southampton UK"
}
],
"family": "Hatchard",
"given": "Parys",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Southampton Clinical Trials Unit University of Southampton Southampton UK"
}
],
"family": "Waugh",
"given": "Robert",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Southampton Clinical Trials Unit University of Southampton Southampton UK"
}
],
"family": "Lawrence",
"given": "Megan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Southampton Clinical Trials Unit University of Southampton Southampton UK"
}
],
"family": "Johnson",
"given": "Lucy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Southampton Clinical Trials Unit University of Southampton Southampton UK"
}
],
"family": "Fines",
"given": "Keira",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7443-4520",
"affiliation": [
{
"name": "University of Liverpool Liverpool UK"
}
],
"authenticated-orcid": false,
"family": "Reynolds",
"given": "Helen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Liverpool University Hospitals NHS Foundation Trust Liverpool UK"
}
],
"family": "Rowland",
"given": "Timothy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Liverpool University Hospitals NHS Foundation Trust Liverpool UK"
}
],
"family": "Crook",
"given": "Rebecca",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Liverpool University Hospitals NHS Foundation Trust Liverpool UK"
}
],
"family": "Okenyi",
"given": "Emmanuel",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8895-5618",
"affiliation": [
{
"name": "Liverpool School of Tropical Medicine Liverpool UK"
}
],
"authenticated-orcid": false,
"family": "Byrne",
"given": "Kelly",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "MRC Biostatistics Unit University of Cambridge Cambridge UK"
}
],
"family": "Mozgunov",
"given": "Pavel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "MRC Biostatistics Unit University of Cambridge Cambridge UK"
}
],
"family": "Jaki",
"given": "Thomas",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2769-0967",
"affiliation": [
{
"name": "University of Liverpool Liverpool UK"
}
],
"authenticated-orcid": false,
"family": "Khoo",
"given": "Saye",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9819-7651",
"affiliation": [
{
"name": "University of Liverpool Liverpool UK"
}
],
"authenticated-orcid": false,
"family": "Owen",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Southampton Clinical Trials Unit University of Southampton Southampton UK"
}
],
"family": "Griffiths",
"given": "Gareth",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Liverpool University Hospitals NHS Foundation Trust Liverpool UK"
},
{
"name": "Liverpool School of Tropical Medicine Liverpool UK"
}
],
"family": "Fletcher",
"given": "Thomas E.",
"sequence": "additional"
},
{
"affiliation": [],
"name": "the AGILE platform",
"sequence": "additional"
}
],
"container-title": "Clinical Pharmacology & Therapeutics",
"container-title-short": "Clin Pharma and Therapeutics",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"ascpt.onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2021,
10,
26
]
],
"date-time": "2021-10-26T20:04:33Z",
"timestamp": 1635278673000
},
"deposited": {
"date-parts": [
[
2023,
11,
7
]
],
"date-time": "2023-11-07T13:38:50Z",
"timestamp": 1699364330000
},
"indexed": {
"date-parts": [
[
2024,
3,
1
]
],
"date-time": "2024-03-01T00:46:34Z",
"timestamp": 1709253994298
},
"is-referenced-by-count": 14,
"issue": "3",
"issued": {
"date-parts": [
[
2021,
11,
13
]
]
},
"journal-issue": {
"issue": "3",
"published-print": {
"date-parts": [
[
2022,
3
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
11,
13
]
],
"date-time": "2021-11-13T00:00:00Z",
"timestamp": 1636761600000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/cpt.2463",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1002/cpt.2463",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://ascpt.onlinelibrary.wiley.com/doi/pdf/10.1002/cpt.2463",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"page": "585-594",
"prefix": "10.1111",
"published": {
"date-parts": [
[
2021,
11,
13
]
]
},
"published-online": {
"date-parts": [
[
2021,
11,
13
]
]
},
"published-print": {
"date-parts": [
[
2022,
3
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1016/S2589-7500(20)30086-8",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_1_1"
},
{
"DOI": "10.1111/bcp.14619",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_2_1"
},
{
"DOI": "10.1038/s41586-020-2575-3",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_3_1"
},
{
"article-title": "Hydroxychloroquine proves ineffective in hamsters and macaques infected with SARS‐CoV‐2",
"author": "Rosenke K.",
"journal-title": "bioRxiv",
"key": "e_1_2_10_4_1"
},
{
"key": "e_1_2_10_5_1",
"unstructured": "Food and Drug Administration Agency. <https://www.accessdata.fda.gov/drugsatfda_docs/nda/2004/21‐497_Alinia_Approv.pdf>. Accessed November 03 2021."
},
{
"DOI": "10.1016/S0065-308X(10)73008-6",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_6_1"
},
{
"DOI": "10.1111/j.1365-2036.2006.03128.x",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_7_1"
},
{
"DOI": "10.1016/j.antiviral.2007.08.005",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_8_1"
},
{
"DOI": "10.1128/AAC.00078-08",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_9_1"
},
{
"DOI": "10.1128/AAC.00707-18",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_10_1"
},
{
"DOI": "10.1093/infdis/jix377",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_11_1"
},
{
"DOI": "10.1111/irv.12446",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_12_1"
},
{
"DOI": "10.1016/j.antiviral.2014.07.014",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_13_1"
},
{
"DOI": "10.1016/S1473-3099(14)70717-0",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_14_1"
},
{
"DOI": "10.1183/13993003.03725-2020",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_15_1"
},
{
"DOI": "10.1016/j.eclinm.2021.100981",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_16_1"
},
{
"DOI": "10.1101/2021.04.19.21255441",
"doi-asserted-by": "crossref",
"key": "e_1_2_10_17_1",
"unstructured": "Rossignol J.F.et al.Early treatment with nitazoxanide prevents worsening of mild and moderate COVID‐19 and subsequent hospitalization. medRxiv Preprint. https://doi.org/https://doi.org/10.1101/2021.04.19.21255441."
},
{
"DOI": "10.1093/jac/dkab189",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_18_1"
},
{
"DOI": "10.1111/bcp.14575",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_19_1"
},
{
"DOI": "10.1517/17425250902988487",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_20_1"
},
{
"key": "e_1_2_10_21_1",
"unstructured": "Riccio A.et al.Impairment of SARS‐CoV‐2 spike glycoprotein maturation and fusion activity by the broad‐spectrum anti‐infective drug nitazoxanide. bioRxiv Preprint.https://doi.org/10.1101/2021.01.12.439201. [e‐pub ahead of print]."
},
{
"DOI": "10.1038/srep27148",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_22_1"
},
{
"DOI": "10.3389/fphar.2019.00051",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_23_1"
},
{
"DOI": "10.1038/s41586-021-03491-6",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_24_1"
},
{
"DOI": "10.1002/cpt.1909",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_25_1"
},
{
"DOI": "10.5414/CPP40221",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_26_1"
},
{
"DOI": "10.1186/s13063-020-04473-1",
"article-title": "AGILE‐ACCORD: a randomized, multicentre, seamless, adaptive phase I/II platform study to determine the optimal dose, safety and efficacy of multiple candidate agents for the treatment of COVID‐19: a structured summary of a study protocol for a randomised platform trial",
"author": "Griffiths G.",
"doi-asserted-by": "crossref",
"first-page": "544",
"journal-title": "Trials",
"key": "e_1_2_10_27_1",
"volume": "21",
"year": "2020"
},
{
"DOI": "10.1111/bcp.14619",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_28_1"
},
{
"DOI": "10.1128/AAC.01918-13",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_29_1"
},
{
"author": "R Core Team",
"key": "e_1_2_10_30_1",
"volume-title": "R: A language and environment for statistical computing",
"year": "2020"
},
{
"key": "e_1_2_10_31_1",
"unstructured": "Borchers H.W.pracma: Practical Numerical Math Functions. R package version 2.2.9 (2019) <https://CRAN.R‐project.org/package=pracma>."
},
{
"DOI": "10.1111/j.1541-0420.2011.01560.x",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_32_1"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_33_1"
},
{
"DOI": "10.1016/j.antiviral.2014.11.010",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_34_1"
},
{
"DOI": "10.1101/2020.06.29.178889",
"doi-asserted-by": "crossref",
"key": "e_1_2_10_35_1",
"unstructured": "Bobrowski T.et al.Discovery of synergistic and antagonistic drug combinations against SARS‐CoV‐2 in vitro. bioRxiv preprint.https://doi.org/10.1101/2020.06.29.178889. [e‐pub ahead of print]."
},
{
"key": "e_1_2_10_36_1",
"unstructured": "National Center for Advancing Translational Sciences Open Data Portal Nitazoxanide(2020) <https://opendata.ncats.nih.gov/covid19/databrowser?q=Nitazoxanide>. Accessed July 20 2021."
},
{
"DOI": "10.1074/jbc.M109.029470",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_37_1"
},
{
"DOI": "10.1002/cpt.2099",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_38_1"
},
{
"DOI": "10.1002/cpt.2210",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_39_1"
},
{
"DOI": "10.1002/cpt.2159",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_40_1"
}
],
"reference-count": 40,
"references-count": 40,
"relation": {
"has-preprint": [
{
"asserted-by": "object",
"id": "10.1101/2021.09.10.21263376",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "https://ascpt.onlinelibrary.wiley.com/doi/10.1002/cpt.2463"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "An Open Label, Adaptive, Phase 1 Trial of High‐Dose Oral Nitazoxanide in Healthy Volunteers: An Antiviral Candidate for SARS‐CoV‐2",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy",
"volume": "111"
}