Use of casirivimab and imdevimab to prevent progression to severe COVID-19
et al., Journal of Integrative Medicine and Public Health, doi:10.4103/JIMPH.JIMPH_23_24, Jul 2024
19th treatment shown to reduce risk in
March 2021, now with p = 0.000095 from 34 studies, recognized in 52 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Prospective study of 169 non-hospitalized mild-to-moderate COVID-19 patients at high risk of progression in India, showing significantly lower progression and faster viral clearance with casirivimab/imdevimab.
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for many omicron variants1-7.
|
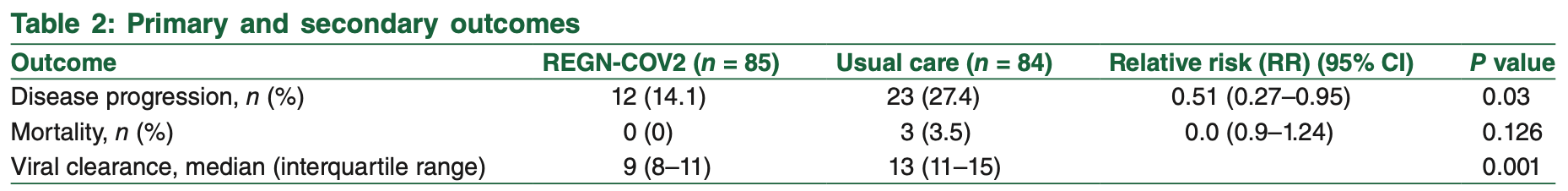
risk of death, 85.8% lower, RR 0.14, p = 0.12, treatment 0 of 85 (0.0%), control 3 of 84 (3.6%), NNT 28, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of progression, 48.4% lower, RR 0.52, p = 0.04, treatment 12 of 85 (14.1%), control 23 of 84 (27.4%), NNT 7.5.
|
|
time to viral-, 30.8% lower, relative time 0.69, p = 0.001, treatment 85, control 84.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
4.
Tatham et al., Lack of Ronapreve (REGN-CoV; casirivimab and imdevimab) virological efficacy against the SARS-CoV 2 Omicron variant (B.1.1.529) in K18-hACE2 mice, bioRxiv, doi:10.1101/2022.01.23.477397.
5.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
Shahnawaz et al., 31 Jul 2024, prospective, India, peer-reviewed, mean age 62.4, 7 authors, study period June 2021 - October 2021.
Contact: naveednazirshah@yahoo.com.
Use of casirivimab and imdevimab to prevent progression to severe COVID-19
Journal of Integrative Medicine and Public Health, doi:10.4103/jimph.jimph_23_24
BACKGROUND: Advanced age and the presence of comorbid illnesses increase the risk of disease progression and death in patients infected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV2). Several neutralizing antibodies against SARS-CoV2 have been developed for the management of mild to moderate coronavirus disease 2019 (COVID-19) at risk of disease progression. This study aimed to evaluate the effect of a combination of casirivimab and imdevimab (REGN-COV2) on disease progression/hospitalization in mild-to-moderate COVID-19.
MATERIALS AND METHODS: It is a prospective interventional study, patients with mild-to-moderate COVID-19 were included in the study. The illnesses with risk factors for disease progression were divided into two groups, one who received a combination of casirivimab and imdevimab (REGN-COV2) cocktail and the other who received usual care. The primary outcome of the study was a comparison of disease progression or hospitalization on day 14 after treatment. Secondary outcomes included viral clearance, mortality, and effect on proinflammatory markers.
RESULTS: A total of 169 patients with mild-to-moderate COVID-19 illness with risk factors for disease progression were included in the study. About 85 patients (50.3%) as cases received REGN-COV2 whereas 84 (49.7%) as control received usual care only. Hospitalization or progression of disease was significantly lower in patients who received REGN-COV2 than in the usual care only [14.12% vs. 27.38%; relative risk (RR) = 0.51, 95% confidence interval (CI) = 0.27-0.95, P = 0.03]. Time taken for viral clearance was again lower in intervention group [median = 9 days, interquartile range (IQR) = [8-11] vs. median =13 days, IQR [10-14], P < 0.001] as was serum levels of C-reactive protein (CRP) and interleukin (IL)-6 significantly reduced in patients who received REGN-COV2 [CRP, median = 11.5 (5.75-34) to 4 (2-9), P = 0.001 and IL-6, median 10 (4-23.5) to 3 (2-7), P = 0.001]. Three patients in the usual care group died due to worsening respiratory failure compared with none in the REGN-COV2 group. Only minor adverse events were noticed in 8.2% (n = 7) patients in the REGN-COV2 group with no life-threatening events observed.
CONCLUSION: In mild-to-moderate COVID-19 patients at high risk of disease progression REGN-COV2 treatment significantly reduced progression of disease and hospitalization without significant adverse events.
Financial support and sponsorship Nil.
Conflicts of interest There are no conflicts of interest.
References
Blanco-Melo, Nilsson-Payan, Liu, Uhl, Hoagland et al., Imbalanced host response to SARS-CoV-2 drives development of COVID-19, Cell
Chen, Qi, Liu, Ling, Qian et al., Clinical progression of patients with COVID-19 in Shanghai, China, J Infect
Clark, Jit, Warren-Gash, Guthrie, Wang et al., Global, regional and national estimates of the population at risk of severe COVID-19 due to underlying health conditions in 2020: A modelling study, Lancet Glob Health
Docherty, Harrison, Green, Hardwick, Pius et al., ISARIC4C investigators. Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO clinical characterization protocol: Prospective observational cohort study, BMJ
Liu, Li, Xu, Wu, Luo et al., Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19, J Clin Virol
Liu, Yan, Wang, Xiang, Le et al., Viral dynamics in mild and severe cases of COVID-19, Lancet Infect Dis
Magleby, Westblade, Trzebucki, Simon, Rajan et al., Impact of SARS COV-2 viral load on risk of intubation and mortality among hospitalized patients with coronavirus disease 2019, Clin Infect Dis
Razonable, Pawlowski, Horo, Arndt, Arndt et al., Casirivimab Imdevimab treatment is associated with reduced rates of hospitalization among high-risk patients with mild to moderate coronavirus disease-19, E-ClinicalMedicine
Renn, Fu, Hu, Hall, Simeonov, Fruitfuls neutralizing antibody pipeline brings hope to defeat SARS-Cov-2, Trends Pharmacol Sci
Richardson, Hirsch, Narasimhan, Crawford, Mcginn et al., Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New City area, JAMA
Sahu, Kampa, Padhi, Panda, C-reactive protein: A promising biomarker for poor prognosis in COVID-19 infection, Clin Chim acta
Shanmugaraj, Siriwattananon, Wangkanont, Phoolcharoen, Perspectives on monoclonal antibody therapy as potential therapeutic intervention for coronavirus disease-19 (COVID-19), Asian Pac J Allergy Immunol
Tabata, Imai, Kawano, Ikeda, Kodama et al., Clinical characteristics of COVID-19 in 104 people with SARS-CoV-2 infection on the Diamond Princess cruise ship: A retrospective analysis, Lancet Infect Dis
Tenforde, Kim, Lindsel, Rose, Shapiro et al., Symptom duration and risk factors for delayed return to usual health among outpatients with cOVID-19 in a multi-state health care systems network-United States, March-June 2020, MMWR Morb Mortal Wkly Rep
Weinreich, Sivapalasingam, Norton, Ali, Gao et al., REGN-COV2, a neutralizing antibody Cocktail, in outpatients with Covid-19, N Engl J Med
Westblade, Brar, Pinheiro, Paidoussis, Rajan et al., SARS-CoV-2 Viral load predicts mortality in patients with and without cancer who are hospitalized with COVID-19, Cancer Cell
DOI record:
{
"DOI": "10.4103/jimph.jimph_23_24",
"ISSN": [
"2949-7248",
"2949-7256"
],
"URL": "http://dx.doi.org/10.4103/JIMPH.JIMPH_23_24",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>BACKGROUND:</jats:title>\n <jats:p>Advanced age and the presence of comorbid illnesses increase the risk of disease progression and death in patients infected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV2). Several neutralizing antibodies against SARS-CoV2 have been developed for the management of mild to moderate coronavirus disease 2019 (COVID-19) at risk of disease progression. This study aimed to evaluate the effect of a combination of casirivimab and imdevimab (REGN-COV2) on disease progression/hospitalization in mild-to-moderate COVID-19.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>MATERIALS AND METHODS:</jats:title>\n <jats:p>It is a prospective interventional study, patients with mild-to-moderate COVID-19 were included in the study. The illnesses with risk factors for disease progression were divided into two groups, one who received a combination of casirivimab and imdevimab (REGN-COV2) cocktail and the other who received usual care. The primary outcome of the study was a comparison of disease progression or hospitalization on day 14 after treatment. Secondary outcomes included viral clearance, mortality, and effect on proinflammatory markers.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>RESULTS:</jats:title>\n <jats:p>A total of 169 patients with mild-to-moderate COVID-19 illness with risk factors for disease progression were included in the study. About 85 patients (50.3%) as cases received REGN-COV2 whereas 84 (49.7%) as control received usual care only. Hospitalization or progression of disease was significantly lower in patients who received REGN-COV2 than in the usual care only [14.12% vs. 27.38%; relative risk (RR) = 0.51, 95% confidence interval (CI) = 0.27−0.95, <jats:italic toggle=\"yes\">P</jats:italic> = 0.03]. Time taken for viral clearance was again lower in intervention group [median = 9 days, interquartile range (IQR) = [8–11] vs. median =13 days, IQR [10–14], <jats:italic toggle=\"yes\">P</jats:italic> < 0.001] as was serum levels of C-reactive protein (CRP) and interleukin (IL)-6 significantly reduced in patients who received REGN-COV2 [CRP, median = 11.5 (5.75–34) to 4 (2–9), <jats:italic toggle=\"yes\">P</jats:italic> = 0.001 and IL-6, median 10 (4–23.5) to 3 (2–7), <jats:italic toggle=\"yes\">P</jats:italic> = 0.001]. Three patients in the usual care group died due to worsening respiratory failure compared with none in the REGN-COV2 group. Only minor adverse events were noticed in 8.2% (<jats:italic toggle=\"yes\">n</jats:italic> = 7) patients in the REGN-COV2 group with no life-threatening events observed.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>CONCLUSION:</jats:title>\n <jats:p>In mild-to-moderate COVID-19 patients at high risk of disease progression REGN-COV2 treatment significantly reduced progression of disease and hospitalization without significant adverse events.</jats:p>\n </jats:sec>",
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"value": "2024-11-07"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"value": "2024-12-16"
}
],
"author": [
{
"affiliation": [
{
"name": "Department of Pulmonary Medicine, Government Medical College, Srinagar, Jammu and Kashmir, India"
}
],
"family": "Shahnawaz",
"given": "Mir",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Pulmonary Medicine, Government Medical College, Srinagar, Jammu and Kashmir, India"
}
],
"family": "Hussain",
"given": "Tajamul",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pulmonary Medicine, Government Medical College, Srinagar, Jammu and Kashmir, India"
}
],
"family": "Firdous",
"given": "Naeem",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pulmonary Medicine, Government Medical College, Srinagar, Jammu and Kashmir, India"
}
],
"family": "Yousoof",
"given": "Mohammad",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pulmonary Medicine, Government Medical College, Srinagar, Jammu and Kashmir, India"
}
],
"family": "Gundroo",
"given": "Hafsa M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Jammu Kashmir Medical Supplies Council Ltd., Srinagar, Jammu and Kashmir, India"
}
],
"family": "Sharma",
"given": "YashPal",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pulmonary Medicine, Government Medical College, Srinagar, Jammu and Kashmir, India"
}
],
"family": "Shah",
"given": "Naveed Nazir",
"sequence": "additional"
}
],
"container-title": "Journal of Integrative Medicine and Public Health",
"container-title-short": "J Integr Med Public Health",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"lww.com",
"ovid.com"
]
},
"created": {
"date-parts": [
[
2025,
1,
20
]
],
"date-time": "2025-01-20T06:00:35Z",
"timestamp": 1737352835000
},
"deposited": {
"date-parts": [
[
2025,
1,
20
]
],
"date-time": "2025-01-20T06:00:40Z",
"timestamp": 1737352840000
},
"indexed": {
"date-parts": [
[
2025,
1,
21
]
],
"date-time": "2025-01-21T05:03:28Z",
"timestamp": 1737435808312,
"version": "3.33.0"
},
"is-referenced-by-count": 0,
"issue": "2",
"issued": {
"date-parts": [
[
2024,
7
]
]
},
"journal-issue": {
"issue": "2",
"published-print": {
"date-parts": [
[
2024
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc-sa/4.0",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
7,
1
]
],
"date-time": "2024-07-01T00:00:00Z",
"timestamp": 1719792000000
}
}
],
"link": [
{
"URL": "https://journals.lww.com/10.4103/JIMPH.JIMPH_23_24",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "276",
"original-title": [],
"page": "94-100",
"prefix": "10.4103",
"published": {
"date-parts": [
[
2024,
7
]
]
},
"published-online": {
"date-parts": [
[
2025,
1,
20
]
]
},
"published-print": {
"date-parts": [
[
2024,
7
]
]
},
"publisher": "Ovid Technologies (Wolters Kluwer Health)",
"reference": [
{
"DOI": "10.1016/S1473-3099(20)30482-5",
"article-title": "Clinical characteristics of COVID-19 in 104 people with SARS-CoV-2 infection on the Diamond Princess cruise ship: A retrospective analysis",
"author": "Tabata",
"doi-asserted-by": "crossref",
"first-page": "1043",
"journal-title": "Lancet Infect Dis",
"key": "CIT0001-20250120",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1136/bmj.m1985",
"article-title": "Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO clinical characterization protocol: Prospective observational cohort study",
"author": "Docherty",
"doi-asserted-by": "crossref",
"first-page": "m1985",
"journal-title": "BMJ",
"key": "CIT0002-20250120",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.04.026",
"article-title": "Imbalanced host response to SARS-CoV-2 drives development of COVID-19",
"author": "Blanco-Melo",
"doi-asserted-by": "crossref",
"first-page": "1036",
"journal-title": "Cell",
"key": "CIT0003-20250120",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1016/j.jinf.2020.03.004",
"article-title": "Clinical progression of patients with COVID-19 in Shanghai, China",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "e1",
"journal-title": "J Infect",
"key": "CIT0004-20250120",
"volume": "80",
"year": "2020"
},
{
"DOI": "10.1016/S1473-3099(20)30232-2",
"article-title": "Viral dynamics in mild and severe cases of COVID-19",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "656",
"journal-title": "Lancet Infect Dis",
"key": "CIT0005-20250120",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1016/j.ccell.2020.09.007",
"article-title": "SARS-CoV-2 Viral load predicts mortality in patients with and without cancer who are hospitalized with COVID-19",
"author": "Westblade",
"doi-asserted-by": "crossref",
"first-page": "661",
"journal-title": "Cancer Cell",
"key": "CIT0006-20250120",
"volume": "38",
"year": "2020"
},
{
"DOI": "10.1016/j.tips.2020.07.004",
"article-title": "Fruitfuls neutralizing antibody pipeline brings hope to defeat SARS- Cov-2",
"author": "Renn",
"doi-asserted-by": "crossref",
"first-page": "815",
"journal-title": "Trends Pharmacol Sci",
"key": "CIT0007-20250120",
"volume": "41",
"year": "2020"
},
{
"article-title": "Perspectives on monoclonal antibody therapy as potential therapeutic intervention for coronavirus disease-19 (COVID-19)",
"author": "Shanmugaraj",
"first-page": "10",
"journal-title": "Asian Pac J Allergy Immunol",
"key": "CIT0008-20250120",
"volume": "38",
"year": "2020"
},
{
"article-title": "An EUA for Casirivimab and Imdevimab for COVID-19",
"first-page": "2012",
"journal-title": "Med Lett Drugs Thers",
"key": "CIT0013-20250120",
"volume": "62",
"year": "2020"
},
{
"DOI": "10.1016/j.eclinm.2021.101102",
"article-title": "Casirivimab Imdevimab treatment is associated with reduced rates of hospitalization among high-risk patients with mild to moderate coronavirus disease-19",
"author": "Razonable",
"doi-asserted-by": "crossref",
"first-page": "101102",
"journal-title": "E-ClinicalMedicine",
"key": "CIT0014-20250120",
"volume": "40",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2035002",
"article-title": "REGN-COV2, a neutralizing antibody Cocktail, in outpatients with Covid-19",
"author": "Weinreich",
"doi-asserted-by": "crossref",
"first-page": "238",
"journal-title": "N Engl J Med",
"key": "CIT0015-20250120",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.15585/mmwr.mm6930e1",
"article-title": "Symptom duration and risk factors for delayed return to usual health among outpatients with cOVID-19 in a multi-state health care systems network–United States, March-June 2020",
"author": "Tenforde",
"doi-asserted-by": "crossref",
"first-page": "993",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "CIT0016-20250120",
"volume": "69",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.6775",
"article-title": "Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New City area",
"author": "Richardson",
"doi-asserted-by": "crossref",
"first-page": "2052",
"journal-title": "JAMA",
"key": "CIT0017-20250120",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1016/S2214-109X(20)30264-3",
"article-title": "Global, regional and national estimates of the population at risk of severe COVID-19 due to underlying health conditions in 2020: A modelling study",
"author": "Clark",
"doi-asserted-by": "crossref",
"first-page": "e1003",
"journal-title": "Lancet Glob Health",
"key": "CIT0018-20250120",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa851",
"article-title": "Impact of SARS COV-2 viral load on risk of intubation and mortality among hospitalized patients with coronavirus disease 2019",
"author": "Magleby",
"doi-asserted-by": "crossref",
"first-page": "e4197",
"journal-title": "Clin Infect Dis",
"key": "CIT0019-20250120",
"volume": "73",
"year": "2020"
},
{
"DOI": "10.1016/j.cca.2020.06.013",
"article-title": "C-reactive protein: A promising biomarker for poor prognosis in COVID-19 infection",
"author": "Sahu",
"doi-asserted-by": "crossref",
"first-page": "91",
"journal-title": "Clin Chim acta",
"key": "CIT0020-20250120",
"volume": "509",
"year": "2020"
},
{
"DOI": "10.1016/j.jcv.2020.104370",
"article-title": "Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "104370",
"journal-title": "J Clin Virol",
"key": "CIT0021-20250120",
"volume": "127",
"year": "2020"
}
],
"reference-count": 17,
"references-count": 17,
"relation": {},
"resource": {
"primary": {
"URL": "https://journals.lww.com/10.4103/JIMPH.JIMPH_23_24"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Use of casirivimab and imdevimab to prevent progression to severe COVID-19",
"type": "journal-article",
"update-policy": "https://doi.org/10.1097/lww.0000000000001000",
"volume": "3"
}
