Early Treatment with BamlanivimabAlone does not Prevent COVID-19 Hospitalization and Its Post-Acute Sequelae. A Real Experience in Umbria, Italy
et al., Mediterranean Journal of Hematology and Infectious Diseases, doi:10.4084/MJHID.2021.061, Oct 2021
25th treatment shown to reduce risk in
May 2021, now with p = 0.00049 from 22 studies, recognized in 11 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 39 patients treated with bamlanivimab in Italy, showing results far below expectations based on the BLAZE1 trial. Authors note that most patients had the alpha variant which does not have the E484K and L452R mutations known to lead to resistance.
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
Schiaroli et al., 29 Oct 2021, retrospective, Italy, peer-reviewed, 7 authors.
EARLY TREATMENT WITH BAMLANIVIMAB ALONE DOES NOT PREVENT COVID-19 HOSPITALIZATION AND ITS POST-ACUTE SEQUELAE. A REAL EXPERIENCE IN UMBRIA, ITALY.
Mediterranean Journal of Hematology and Infectious Diseases, doi:10.4084/mjhid.2021.061
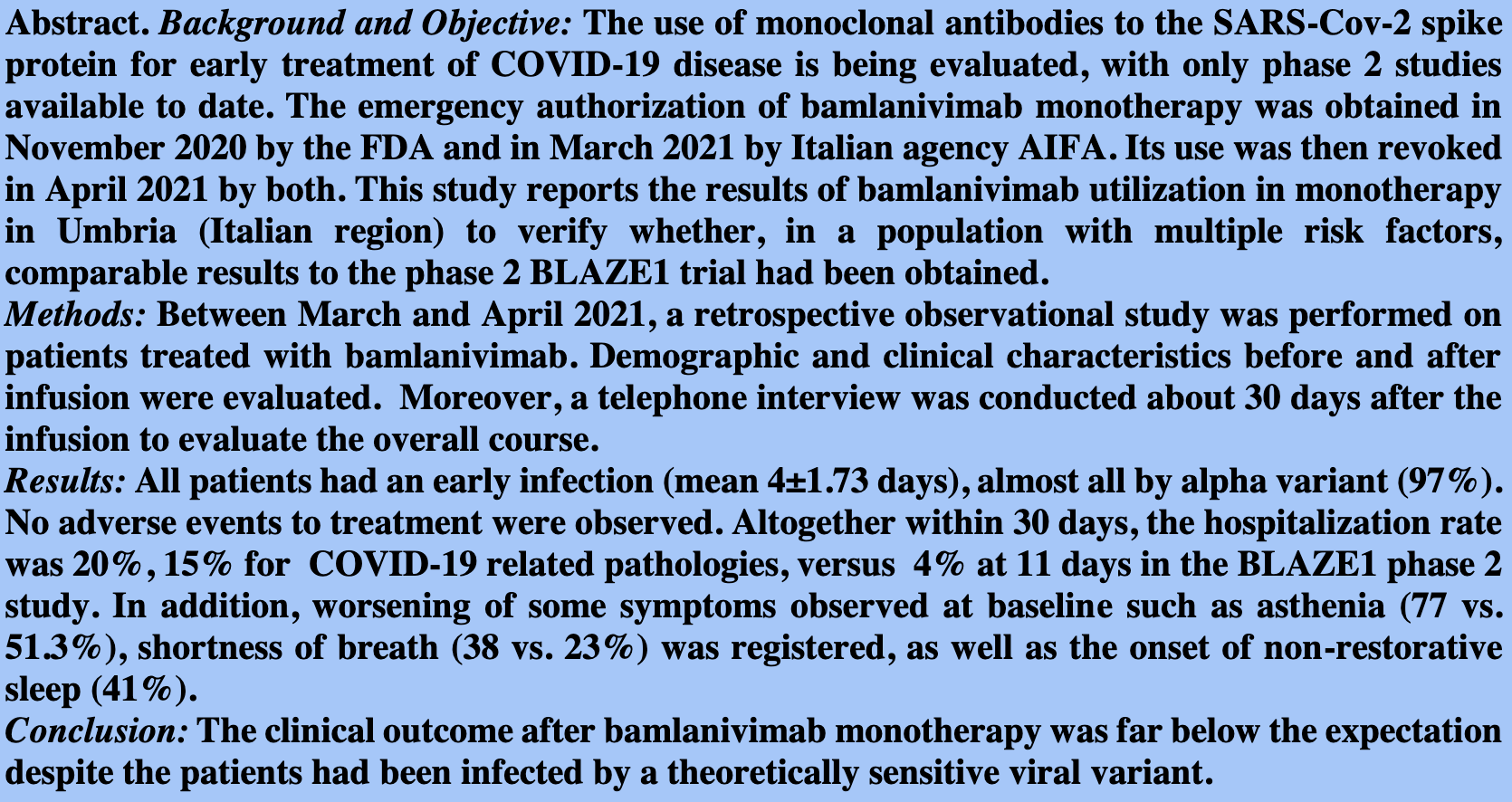
Background and Objective: The use of monoclonal antibodies to the SARS-Cov-2 spike protein for early treatment of COVID-19 disease is being evaluated, with only phase 2 studies available to date. The emergency authorization of bamlanivimab monotherapy was obtained in November 2020 by the FDA and in March 2021 by Italian agency AIFA. Its use was then revoked in April 2021 by both. This study reports the results of bamlanivimab utilization in monotherapy in Umbria (Italian region) to verify whether, in a population with multiple risk factors, comparable results to the phase 2 BLAZE1 trial had been obtained. Methods: Between March and April 2021, a retrospective observational study was performed on patients treated with bamlanivimab. Demographic and clinical characteristics before and after infusion were evaluated. Moreover, a telephone interview was conducted about 30 days after the infusion to evaluate the overall course.
Results: All patients had an early infection (mean 4±1.73 days), almost all by alpha variant (97%). No adverse events to treatment were observed. Altogether within 30 days, the hospitalization rate was 20%, 15% for COVID-19 related pathologies, versus 4% at 11 days in the BLAZE1 phase 2 study. In addition, worsening of some symptoms observed at baseline such as asthenia (77 vs. 51.3%), shortness of breath (38 vs. 23%) was registered, as well as the onset of non-restorative sleep (41%).
Conclusion: The clinical outcome after bamlanivimab monotherapy was far below the expectation despite the patients had been infected by a theoretically sensitive viral variant.
References
Burak Dirim, Demir, Yadigar, Garayeva, Parmaksiz et al., COVID-19 in chronic kidney disease: a retrospective, propensity score-matched cohort study, Int Urol Nephrol, doi:10.1007/s11255-021-02783-0
Chen, Nirula, Heller, Gottlieb, Boscia et al., BLAZE-1 Investigators. SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2029849
Jones, Brown-Augsburger, Corbett, Westendorf, Davies et al., The nutralizing antibody, LY-CoV555, protects against SARS-CoV-2 infection in nonhuman primates, Sci Transl Med, doi:10.1126/scitranslmed.abf1906
Nalbandian, Sehgal, Gupta, Madhavan, Mcgroder et al., Postacute COVID-19 syndrome, Nat Med, doi:10.1038/s41591-021-01283-z
Ramos-Yataco, Meza, Cecilia Farfán-García, Ortega-Rojas, Salinas-Mamani et al., DKA in patients with pre-existing type 2 diabetes mellitus related to COVID-19: a case series, Endocrinol Diabetes Metab Case Rep, doi:10.1530/EDM-20-0148
Widera, Wilhelm, Hoehl, Pallas, Kohmer et al., Bamlanivimab does not neutralize two SARS-CoV-2 variants carrying E484K in vitro medRxiv preprint, doi:10.1101/2021.02.24.21252372
DOI record:
{
"DOI": "10.4084/mjhid.2021.061",
"ISSN": [
"2035-3006"
],
"URL": "http://dx.doi.org/10.4084/MJHID.2021.061",
"abstract": "<jats:p>Background and Objective
\nThe use of monoclonal antibodies to the SARS-Cov-2 spike protein for early treatment of COVID-19 disease is being evaluated, with only phase 2 studies available, to date.
\nEmergency authorization of bamlanivimab monotherapy was get in November 2020 by the FDA and in March 2021 by italian agency AIFA. Its use was then revoked in April 2021 by both.
\nThis study reports the results of bamlanivimab utilization in monotherapy in Umbria (Italian region), in order to verify whether, in a population with multiple risk factors, comparable results to phase 2 BLAZE1 trial had been obtained.
\nMethods
\nRetrospective observational study, between March and April 2021, in patients treated with bamlanivimab was performed. Demographic and clinical characteristics before and after infusion were evaluated. Moreover, a telephone interview about 30 days after the infusion was carried out to evaluate the overall course.
\nResults
\nAll patients had an early infection (mean 4±1.73 days), almost all by alpha variant (97%).
\nNo adverse events to treatment were observed. Altogether within 30 days, the hospitalization rate was 20%, 15% for COVID-19 related pathologies , versus 4% at 11 days in BLAZE1 phase 2 study. Worsening of some symptoms observed at baseline such as asthenia (77 vs 51.3%), shortness of breath (38 vs 23%) was registered, as well as the onset of non-restorative sleep (41%).
\nConclusions
\nThe clinical outcome after bamlanivimab monotherapy was far below the expectation despite the patients had been infected by a theoretically sensitive viral variant.</jats:p>",
"author": [
{
"affiliation": [],
"family": "Schiaroli",
"given": "Elisabetta",
"sequence": "first"
},
{
"affiliation": [],
"family": "De Socio",
"given": "Giuseppe Vittorio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Martinelli",
"given": "Laura Martinelli",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Malincarne",
"given": "Lisa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Savoia",
"given": "Martina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Spinelli",
"given": "Anna Laura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Francisci",
"given": "Daniela",
"sequence": "additional"
}
],
"container-title": "Mediterranean Journal of Hematology and Infectious Diseases",
"container-title-short": "Mediterr J Hematol Infect Dis",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
10,
31
]
],
"date-time": "2021-10-31T17:34:47Z",
"timestamp": 1635701687000
},
"deposited": {
"date-parts": [
[
2021,
10,
31
]
],
"date-time": "2021-10-31T17:34:48Z",
"timestamp": 1635701688000
},
"indexed": {
"date-parts": [
[
2022,
4,
4
]
],
"date-time": "2022-04-04T04:15:37Z",
"timestamp": 1649045737861
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2021,
10,
29
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2021,
1,
1
]
]
}
},
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc/4.0",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
10,
29
]
],
"date-time": "2021-10-29T00:00:00Z",
"timestamp": 1635465600000
}
}
],
"link": [
{
"URL": "https://www.mjhid.org/index.php/mjhid/article/download/4690/4077",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.mjhid.org/index.php/mjhid/article/download/4690/4083",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.mjhid.org/index.php/mjhid/article/download/4690/4077",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "2554",
"original-title": [],
"page": "e2021061",
"prefix": "10.4084",
"published": {
"date-parts": [
[
2021,
10,
29
]
]
},
"published-online": {
"date-parts": [
[
2021,
10,
29
]
]
},
"publisher": "Institute of Hematology, Catholic University",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mjhid.org/index.php/mjhid/article/view/4690"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "EARLY TREATMENT WITH BAMLANIVIMAB ALONE DOES NOT PREVENT COVID-19 HOSPITALIZATION AND ITS POST-ACUTE SEQUELAE. A REAL EXPERIENCE IN UMBRIA, ITALY.",
"type": "journal-article",
"volume": "13"
}
