Streptococcus salivarius Probiotics to Prevent Acute Otitis Media in Children
et al., JAMA Network Open, doi:10.1001/jamanetworkopen.2023.40608, Nov 2023
Probiotics for COVID-19
20th treatment shown to reduce risk in
March 2021, now with p = 0.00000044 from 29 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
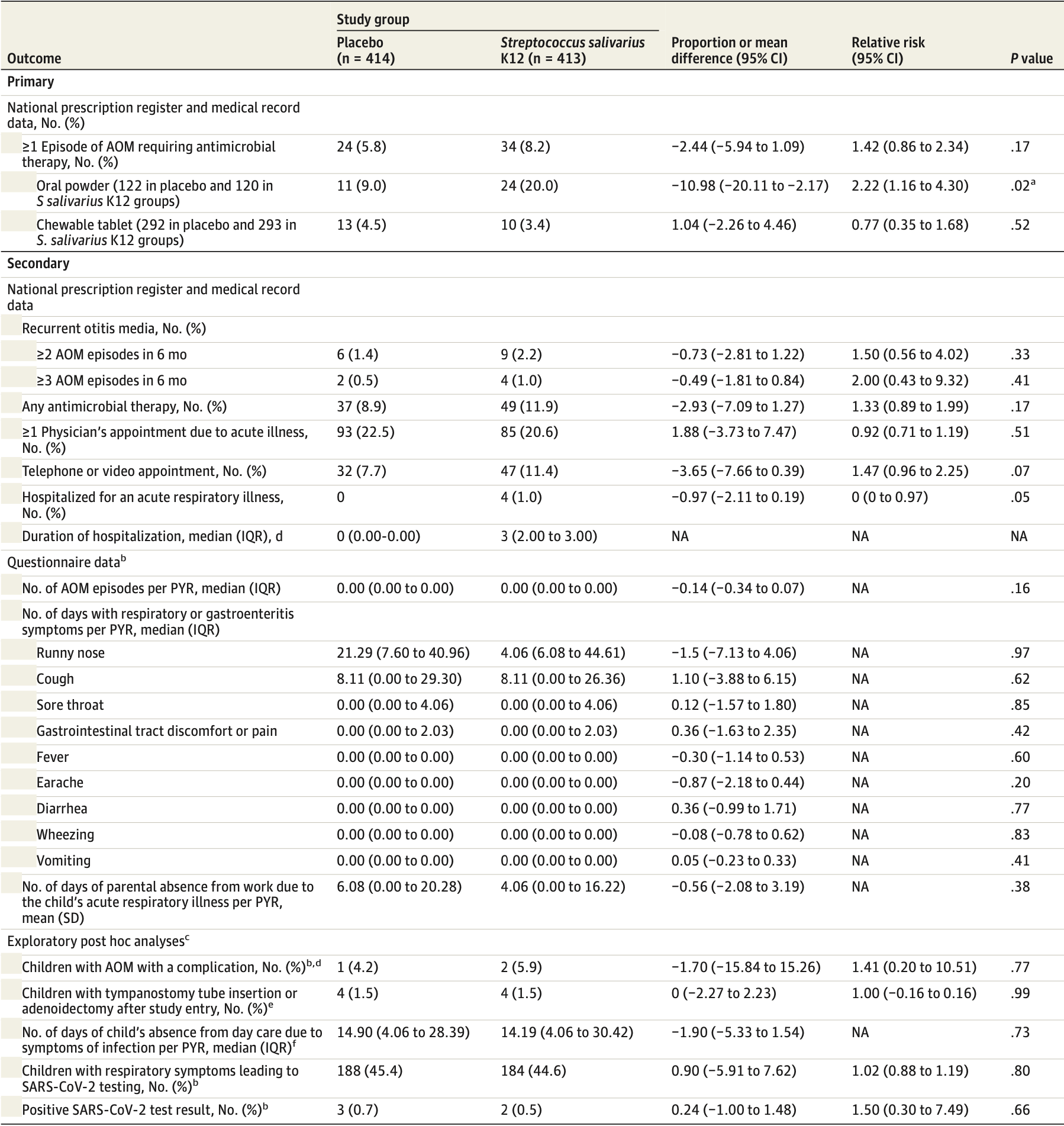
RCT 827 children aged 1-6 years in daycare in Finland analyzing the effectiveness of daily Streptococcus salivarius K12 oral probiotic use for 6 months in preventing acute otitis media (AOM). The probiotic group did not have a significantly lower rate of AOM requiring antibiotics compared to placebo. A secondary outcome shows no significant difference in COVID-19, with only 2 and 3 cases in the treatment and placebo groups.
Probiotic efficacy depends on the specific strains used. Specific microbes may decrease or increase COVID-19 risk1.
Although the 33% fewer cases is not statistically significant, it is consistent with the significant 36% fewer cases [7‑55%] from meta-analysis of the 9 cases results to date.
|
risk of case, 33.2% lower, RR 0.67, p = 1.00, treatment 2 of 413 (0.5%), control 3 of 414 (0.7%), NNT 416.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Sarlin et al., 2 Nov 2023, Double Blind Randomized Controlled Trial, placebo-controlled, Finland, peer-reviewed, 7 authors, study period 1 August, 2020 - 31 May, 2021.
Streptococcus salivarius Probiotics to Prevent Acute Otitis Media in Children
JAMA Network Open, doi:10.1001/jamanetworkopen.2023.40608
IMPORTANCE New approaches for the prevention of acute otitis media (AOM), the most common reason for antibiotic use in children, are needed. OBJECTIVE To assess the efficacy of the Streptococcus salivarius K12 oral probiotics in the primary prevention of AOM. DESIGN, SETTING, AND PARTICIPANTS This double-blind, randomized placebo-controlled clinical trial was conducted from August 1, 2020, to May 31, 2021, at 50 day care centers in the Oulu region of Finland. A total of 827 children aged 1 to 6 years attending day care were included. The exclusion criteria consisted of ongoing antimicrobial prophylaxis or immunodeficiency. The follow-up time was 6 months and was completed on May 31, 2021. Data were analyzed from October 24, 2022, to September 16, 2023, based on intention to treat. INTERVENTION Eligible participants were randomly allocated to receive 1 daily dose of a S salivarius K12 product or placebo every evening for 6 months. A daily dose was defined as 1 sachet of soluble oral powder for children younger than 3 years or 1 chewable tablet for children 3 years or older containing 1 × 10 9 colony-forming units of S salivarius K12.
MAIN OUTCOMES AND MEASURES The primary outcome was the proportion of children with at least 1 episode of AOM requiring antimicrobial therapy within 6 months of randomization. All physician visits and purchases of antimicrobial drugs were retrieved from the electronic national medical record and prescription register. The primary outcome was met if the legal guardian had purchased an antimicrobial prescription for AOM.
RESULTS A total of 827 children with a mean (SD) age of 4.1 (1.6) years (433 boys [52.4%]) were randomized to S salivarius K12 oral products (n = 413) or placebo (n = 414). Thirty-four children (8.2%) in the S salivarius group and 24 children (5.8%) in the placebo group experienced at least 1 episode of AOM requiring antimicrobial therapy during the 6-month follow-up period (relative risk, 1.42 [95% CI, 0.86-2.34]; proportion difference, -2.44% [95% CI, -5.94% to 1.09%]; P = .17). Time to first AOM episode did not differ between the groups (174 [95% CI, 171-177] days in the S salivarius group vs 176 [95% CI, 173-179] days in the placebo group; P = .18).
CONCLUSIONS AND RELEVANCE In this randomized placebo-controlled clinical trial, the daily use of the S salivarius K12 products for 6 months did not reduce the occurrence of AOM. New approaches for primary prevention of AOM among children are needed.
Additional Contributions: We thank the staff at day care centers in the cities of Oulu, Kempele, Tyrnävä, Liminka, and Muhos, Finland. Aino Ruohola, MD, PhD, University of Turku, Turku, Finland, provided discussions and comments throughout the study and Leena Okkonen, RN, Oulu University Hospital Oulu, Oulu, Finland, helped in the project in her role as study nurse; they were not compensated financially for their contributions.
References
Armitage, Berry, Matthews, JAMA Network Open | Pediatrics Effectiveness of S salivarius Probiotics to Prevent Acute Otitis Media in Children JAMA Network Open
Arola, Ziegler, Ruuskanen, Mertsola, Näntö-Salonen et al., Rhinovirus in acute otitis media, J Pediatr, doi:10.1016/S0022-3476(88)80380-9
Chonmaitree, Jennings, Golovko, Nasopharyngeal microbiota in infants and changes during viral upper respiratory tract infection and acute otitis media, PLoS One, doi:10.1371/journal.pone.0180630
Fireman, Black, Shinefield, Lee, Lewis et al., Impact of the pneumococcal conjugate vaccine on otitis media, Pediatr Infect Dis J, doi:10.1097/00006454-200301000-00006
Franch-Llasat, Bellaubí-Pallarés, Mo, Chamarro-Martí, García-Rodríguez et al., Pneumococcal meningitis secondary to otitis media in two patients with COVID-19 Omicron variant, Int J Emerg Med, doi:10.1186/s12245-022-00448-y
Hatakka, Blomgren, Pohjavuori, Treatment of acute otitis media with probiotics in otitis-prone children-a double-blind, placebo-controlled randomised study, Clin Nutr, doi:10.1016/j.clnu.2007.01.003
Heikkinen, Ruuskanen, Waris, Ziegler, Arola et al., Influenza vaccination in the prevention of acute otitis media in children, AJDC, doi:https://jama.jamanetwork.com/article.aspx?doi=10.1001/archpedi.1991.02160040103017&utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jamanetworkopen.2023.40608
Hullegie, Schilder, Marchisio, A strong decline in the incidence of childhood otitis media during the COVID-19 pandemic in the Netherlands, Front Cell Infect Microbiol, doi:10.3389/fcimb.2021.768377
Jokinen, Palmu, Kilpi, Acute otitis media replacement and recurrence in the Finnish Otitis Media Vaccine trial, Clin Infect Dis, doi:10.1093/cid/cis799
Kadambari, Goldacre, Morris, Goldacre, Pollard, Indirect effects of the COVID-19 pandemic on childhood infection in England: population based observational study, BMJ, doi:10.1136/bmj-2021-067519
Karppinen, Toivonen, Schuez-Havupalo, Effectiveness of the ten-valent pneumococcal Haemophilus influenzae protein D conjugate vaccine (PHiD-CV10) against all respiratory tract infections in children under two years of age, Vaccine, doi:10.1016/j.vaccine.2019.04.026
Kuitunen, Artama, Haapanen, Renko, Rhinovirus spread in children during the COVID-19 pandemic despite social restrictions-a nationwide register study in Finland, J Med Virol, doi:10.1002/jmv.27180
Kuitunen, Artama, Mäkelä, Backman, Heiskanen-Kosma et al., Effect of social distancing due to the COVID-19 pandemic on the incidence of viral respiratory tract infections in children in Finland during early 2020, Pediatr Infect Dis J, doi:10.1097/INF.0000000000002845
Kuitunen, Renko, Antibiotic prescriptions during the first 2 years of the COVID-19 pandemic in Finnish children, Acta Paediatr, doi:10.1111/apa.16515
Kumpu, Kekkonen, Kautiainen, Milk containing probiotic Lactobacillus rhamnosus GG and respiratory illness in children: a randomized, double-blind, placebo-controlled trial, Eur J Clin Nutr, doi:10.1038/ejcn.2012.62
Lehtoranta, Pitkäranta, Korpela, Probiotics in respiratory virus infections, Eur J Clin Microbiol Infect Dis, doi:10.1007/s10096-014-2086-y
Lévesque, Lamothe, Frenette, Coaggregation of Streptococcus salivarius with periodontopathogens: evidence for involvement of fimbriae in the interaction with Prevotella intermedia, Oral Microbiol Immunol, doi:10.1034/j.1399-302X.2003.00085.x
Marchisio, Santagati, Scillato, Streptococcus salivarius 24SMB administered by nasal spray for the prevention of acute otitis media in otitis-prone children, Eur J Clin Microbiol Infect Dis, doi:10.1007/s10096-015-2491-x
Marom, Pitaro, Shah, Otitis media practice during the COVID-19 pandemic, Front Cell Infect Microbiol, doi:10.3389/fcimb.2021.749911
Marom, Schwarz, Gluck, Ginzburg, Tamir, Trends in pediatric acute otitis media burden during the first COVID-19 year, Otol Neurotol, doi:10.1097/MAO.0000000000003581
Norhayati, Ho, Azman, Influenza vaccines for preventing acute otitis media in infants and children, Cochrane Database Syst Rev, doi:10.1002/14651858.CD010089.pub3
Pierro, Colombo, Giuliani, Effect of administration of Streptococcus salivarius K12 on the occurrence of streptococcal pharyngo-tonsillitis, scarlet fever and acute otitis media in 3 years old children, Eur Rev Med Pharmacol Sci
Pierro, Pasquale, Cicco, Oral use of Streptococcus salivarius K12 in children with secretory otitis media: preliminary results of a pilot, uncontrolled study, Int J Gen Med, doi:10.2147/IJGM.S92488
Power, Burton, Chilcott, Dawes, Tagg, Preliminary investigations of the colonisation of upper respiratory tract tissues of infants using a paediatric formulation of the oral probiotic Streptococcus salivarius K12, Eur J Clin Microbiol Infect Dis, doi:10.1007/s10096-008-0569-4
Roos, Håkansson, Holm, Effect of recolonisation with "interfering" alpha streptococci on recurrences of acute and secretory otitis media in children: randomised placebo controlled trial, BMJ, doi:10.1136/bmj.322.7280.210
Sarlin, Tejesvi, Turunen, Impact of Streptococcus salivarius K12 on nasopharyngeal and saliva microbiome: a randomized controlled trial, Pediatr Infect Dis J, doi:10.1097/INF.0000000000003016
Services, None
Tapiovaara, Lehtoranta, Swanljung, Lactobacillus rhamnosus GG in the middle ear after randomized, double-blind, placebo-controlled oral administration, Int J Pediatr Otorhinolaryngol, doi:10.1016/j.ijporl.2014.07.011
Vaz, Kleinman, Raebel, Recent trends in outpatient antibiotic use in children, Pediatrics, doi:10.1542/peds.2013-2903
Vesikari, Forsten, Seppä, Effectiveness of the 10-valent pneumococcal nontypeable Haemophilus influenzae protein D-conjugated vaccine (PHiD-CV) against carriage and acute otitis media-a double-blind randomized clinical trial in Finland, J Pediatric Infect Dis Soc, doi:10.1093/jpids/piw010
Zupancic, Kriksic, Kovacevic, Kovacevic, Influence of oral probiotic Streptococcus salivarius K12 on ear and oral cavity health in humans: a systematic review, Probiotics Antimicrob Proteins, doi:10.1007/s12602-017-9261-2
DOI record:
{
"DOI": "10.1001/jamanetworkopen.2023.40608",
"ISSN": [
"2574-3805"
],
"URL": "http://dx.doi.org/10.1001/jamanetworkopen.2023.40608",
"abstract": "<jats:sec><jats:title>Importance</jats:title><jats:p>New approaches for the prevention of acute otitis media (AOM), the most common reason for antibiotic use in children, are needed.</jats:p></jats:sec><jats:sec><jats:title>Objective</jats:title><jats:p>To assess the efficacy of the <jats:italic>Streptococcus salivarius</jats:italic> K12 oral probiotics in the primary prevention of AOM.</jats:p></jats:sec><jats:sec><jats:title>Design, Setting, and Participants</jats:title><jats:p>This double-blind, randomized placebo-controlled clinical trial was conducted from August 1, 2020, to May 31, 2021, at 50 day care centers in the Oulu region of Finland. A total of 827 children aged 1 to 6 years attending day care were included. The exclusion criteria consisted of ongoing antimicrobial prophylaxis or immunodeficiency. The follow-up time was 6 months and was completed on May 31, 2021. Data were analyzed from October 24, 2022, to September 16, 2023, based on intention to treat.</jats:p></jats:sec><jats:sec><jats:title>Intervention</jats:title><jats:p>Eligible participants were randomly allocated to receive 1 daily dose of a <jats:italic>S salivarius</jats:italic> K12 product or placebo every evening for 6 months. A daily dose was defined as 1 sachet of soluble oral powder for children younger than 3 years or 1 chewable tablet for children 3 years or older containing 1 × 10<jats:sup>9</jats:sup> colony-forming units of <jats:italic>S salivarius</jats:italic> K12.</jats:p></jats:sec><jats:sec><jats:title>Main Outcomes and Measures</jats:title><jats:p>The primary outcome was the proportion of children with at least 1 episode of AOM requiring antimicrobial therapy within 6 months of randomization. All physician visits and purchases of antimicrobial drugs were retrieved from the electronic national medical record and prescription register. The primary outcome was met if the legal guardian had purchased an antimicrobial prescription for AOM.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>A total of 827 children with a mean (SD) age of 4.1 (1.6) years (433 boys [52.4%]) were randomized to <jats:italic>S salivarius</jats:italic> K12 oral products (n = 413) or placebo (n = 414). Thirty-four children (8.2%) in the <jats:italic>S salivarius</jats:italic> group and 24 children (5.8%) in the placebo group experienced at least 1 episode of AOM requiring antimicrobial therapy during the 6-month follow-up period (relative risk, 1.42 [95% CI, 0.86-2.34]; proportion difference, −2.44% [95% CI, −5.94% to 1.09%]; <jats:italic>P</jats:italic> = .17). Time to first AOM episode did not differ between the groups (174 [95% CI, 171-177] days in the <jats:italic>S salivarius</jats:italic> group vs 176 [95% CI, 173-179] days in the placebo group; <jats:italic>P</jats:italic> = .18).</jats:p></jats:sec><jats:sec><jats:title>Conclusions and Relevance</jats:title><jats:p>In this randomized placebo-controlled clinical trial, the daily use of the <jats:italic>S salivarius</jats:italic> K12 products for 6 months did not reduce the occurrence of AOM. New approaches for primary prevention of AOM among children are needed.</jats:p></jats:sec><jats:sec><jats:title>Trial Registration</jats:title><jats:p>ClinicalTrialsRegister.eu Identifier: <jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"uri\" xlink:href=\"https://www.clinicaltrialsregister.eu/ctr-search/search?query=2020-001076-14\">2020-001076-14</jats:ext-link></jats:p></jats:sec>",
"author": [
{
"affiliation": [
{
"name": "Department of Pediatrics and Adolescent Medicine and Medical Research Center, Oulu University Hospital, Oulu, Finland"
},
{
"name": "Research Unit of Clinical Medicine, University of Oulu, Oulu, Finland"
}
],
"family": "Sarlin",
"given": "Suvi",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Pediatrics and Adolescent Medicine and Medical Research Center, Oulu University Hospital, Oulu, Finland"
},
{
"name": "Research Unit of Clinical Medicine, University of Oulu, Oulu, Finland"
}
],
"family": "Koskela",
"given": "Ulla",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pediatrics and Adolescent Medicine and Medical Research Center, Oulu University Hospital, Oulu, Finland"
},
{
"name": "Research Unit of Clinical Medicine, University of Oulu, Oulu, Finland"
}
],
"family": "Honkila",
"given": "Minna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Paediatrics and Adolescent Medicine, Turku University Hospital and University of Turku, Turku, Finland"
}
],
"family": "Tähtinen",
"given": "Paula A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Research Unit of Clinical Medicine, University of Oulu, Oulu, Finland"
},
{
"name": "Research Service Unit, Oulu University Hospital, Oulu, Finland"
}
],
"family": "Pokka",
"given": "Tytti",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pediatrics, Kuopio University Hospital and University of Eastern Finland, Kuopio, Finland"
}
],
"family": "Renko",
"given": "Marjo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pediatrics and Adolescent Medicine and Medical Research Center, Oulu University Hospital, Oulu, Finland"
},
{
"name": "Research Unit of Clinical Medicine, University of Oulu, Oulu, Finland"
}
],
"family": "Tapiainen",
"given": "Terhi",
"sequence": "additional"
}
],
"container-title": "JAMA Network Open",
"container-title-short": "JAMA Netw Open",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
11,
2
]
],
"date-time": "2023-11-02T15:36:01Z",
"timestamp": 1698939361000
},
"deposited": {
"date-parts": [
[
2023,
11,
2
]
],
"date-time": "2023-11-02T15:36:05Z",
"timestamp": 1698939365000
},
"indexed": {
"date-parts": [
[
2023,
11,
3
]
],
"date-time": "2023-11-03T00:55:50Z",
"timestamp": 1698972950666
},
"is-referenced-by-count": 0,
"issue": "11",
"issued": {
"date-parts": [
[
2023,
11,
2
]
]
},
"journal-issue": {
"issue": "11",
"published-print": {
"date-parts": [
[
2023,
11,
1
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://jamanetwork.com/journals/jamanetworkopen/articlepdf/2811238/sarlin_2023_oi_231183_1698093542.53749.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "10",
"original-title": [],
"page": "e2340608",
"prefix": "10.1001",
"published": {
"date-parts": [
[
2023,
11,
2
]
]
},
"published-online": {
"date-parts": [
[
2023,
11,
2
]
]
},
"publisher": "American Medical Association (AMA)",
"reference": [
{
"DOI": "10.1542/peds.2013-2903",
"article-title": "Recent trends in outpatient antibiotic use in children.",
"author": "Vaz",
"doi-asserted-by": "publisher",
"first-page": "375",
"issue": "3",
"journal-title": "Pediatrics",
"key": "zoi231183r1",
"volume": "133",
"year": "2014"
},
{
"DOI": "10.1093/cid/cis799",
"article-title": "Acute otitis media replacement and recurrence in the Finnish Otitis Media Vaccine trial.",
"author": "Jokinen",
"doi-asserted-by": "publisher",
"first-page": "1673",
"issue": "12",
"journal-title": "Clin Infect Dis",
"key": "zoi231183r2",
"volume": "55",
"year": "2012"
},
{
"DOI": "10.1097/00006454-200301000-00006",
"article-title": "Impact of the pneumococcal conjugate vaccine on otitis media.",
"author": "Fireman",
"doi-asserted-by": "publisher",
"first-page": "10",
"issue": "1",
"journal-title": "Pediatr Infect Dis J",
"key": "zoi231183r3",
"volume": "22",
"year": "2003"
},
{
"DOI": "10.1016/j.vaccine.2019.04.026",
"article-title": "Effectiveness of the ten-valent pneumococcal Haemophilus influenzae protein D conjugate vaccine (PHiD-CV10) against all respiratory tract infections in children under two years of age.",
"author": "Karppinen",
"doi-asserted-by": "publisher",
"first-page": "2935",
"issue": "22",
"journal-title": "Vaccine",
"key": "zoi231183r4",
"volume": "37",
"year": "2019"
},
{
"DOI": "10.1093/jpids/piw010",
"article-title": "Effectiveness of the 10-valent pneumococcal nontypeable Haemophilus influenzae protein D–conjugated vaccine (PHiD-CV) against carriage and acute otitis media—a double-blind randomized clinical trial in Finland.",
"author": "Vesikari",
"doi-asserted-by": "publisher",
"first-page": "237",
"issue": "3",
"journal-title": "J Pediatric Infect Dis Soc",
"key": "zoi231183r5",
"volume": "5",
"year": "2016"
},
{
"DOI": "10.1002/14651858.CD010089.pub3",
"article-title": "Influenza vaccines for preventing acute otitis media in infants and children.",
"author": "Norhayati",
"doi-asserted-by": "publisher",
"issue": "10",
"journal-title": "Cochrane Database Syst Rev",
"key": "zoi231183r6",
"volume": "10",
"year": "2017"
},
{
"DOI": "10.1001/archpedi.1991.02160040103017",
"article-title": "Influenza vaccination in the prevention of acute otitis media in children.",
"author": "Heikkinen",
"doi-asserted-by": "publisher",
"first-page": "445",
"issue": "4",
"journal-title": "AJDC",
"key": "zoi231183r7",
"volume": "145",
"year": "1991"
},
{
"DOI": "10.1016/j.ijporl.2014.07.011",
"article-title": "Lactobacillus rhamnosus GG in the middle ear after randomized, double-blind, placebo-controlled oral administration.",
"author": "Tapiovaara",
"doi-asserted-by": "publisher",
"first-page": "1637",
"issue": "10",
"journal-title": "Int J Pediatr Otorhinolaryngol",
"key": "zoi231183r8",
"volume": "78",
"year": "2014"
},
{
"DOI": "10.1007/s10096-014-2086-y",
"article-title": "Probiotics in respiratory virus infections.",
"author": "Lehtoranta",
"doi-asserted-by": "publisher",
"first-page": "1289",
"issue": "8",
"journal-title": "Eur J Clin Microbiol Infect Dis",
"key": "zoi231183r9",
"volume": "33",
"year": "2014"
},
{
"DOI": "10.1371/journal.pone.0180630",
"article-title": "Nasopharyngeal microbiota in infants and changes during viral upper respiratory tract infection and acute otitis media.",
"author": "Chonmaitree",
"doi-asserted-by": "publisher",
"issue": "7",
"journal-title": "PLoS One",
"key": "zoi231183r10",
"volume": "12",
"year": "2017"
},
{
"DOI": "10.1136/bmj.322.7280.210",
"article-title": "Effect of recolonisation with “interfering” alpha streptococci on recurrences of acute and secretory otitis media in children: randomised placebo controlled trial.",
"author": "Roos",
"doi-asserted-by": "publisher",
"first-page": "210",
"issue": "7280",
"journal-title": "BMJ",
"key": "zoi231183r11",
"volume": "322",
"year": "2001"
},
{
"DOI": "10.1007/s10096-008-0569-4",
"article-title": "Preliminary investigations of the colonisation of upper respiratory tract tissues of infants using a paediatric formulation of the oral probiotic Streptococcus salivarius K12.",
"author": "Power",
"doi-asserted-by": "publisher",
"first-page": "1261",
"issue": "12",
"journal-title": "Eur J Clin Microbiol Infect Dis",
"key": "zoi231183r12",
"volume": "27",
"year": "2008"
},
{
"DOI": "10.1097/INF.0000000000003016",
"article-title": "Impact of Streptococcus salivarius K12 on nasopharyngeal and saliva microbiome: a randomized controlled trial.",
"author": "Sarlin",
"doi-asserted-by": "publisher",
"first-page": "394",
"issue": "5",
"journal-title": "Pediatr Infect Dis J",
"key": "zoi231183r13",
"volume": "40",
"year": "2021"
},
{
"DOI": "10.1007/s12602-017-9261-2",
"article-title": "Influence of oral probiotic Streptococcus salivarius K12 on ear and oral cavity health in humans: a systematic review.",
"author": "Zupancic",
"doi-asserted-by": "publisher",
"first-page": "102",
"issue": "2",
"journal-title": "Probiotics Antimicrob Proteins",
"key": "zoi231183r14",
"volume": "9",
"year": "2017"
},
{
"DOI": "10.1034/j.1399-302X.2003.00085.x",
"article-title": "Coaggregation of Streptococcus salivarius with periodontopathogens: evidence for involvement of fimbriae in the interaction with Prevotella intermedia.",
"author": "Lévesque",
"doi-asserted-by": "publisher",
"first-page": "333",
"issue": "5",
"journal-title": "Oral Microbiol Immunol",
"key": "zoi231183r15",
"volume": "18",
"year": "2003"
},
{
"DOI": "10.1038/ejcn.2012.62",
"article-title": "Milk containing probiotic Lactobacillus rhamnosus GG and respiratory illness in children: a randomized, double-blind, placebo-controlled trial.",
"author": "Kumpu",
"doi-asserted-by": "publisher",
"first-page": "1020",
"issue": "9",
"journal-title": "Eur J Clin Nutr",
"key": "zoi231183r16",
"volume": "66",
"year": "2012"
},
{
"DOI": "10.1097/INF.0000000000002845",
"article-title": "Effect of social distancing due to the COVID-19 pandemic on the incidence of viral respiratory tract infections in children in Finland during early 2020.",
"author": "Kuitunen",
"doi-asserted-by": "publisher",
"first-page": "e423",
"issue": "12",
"journal-title": "Pediatr Infect Dis J",
"key": "zoi231183r19",
"volume": "39",
"year": "2020"
},
{
"DOI": "10.2147/IJGM",
"article-title": "Oral use of Streptococcus salivarius K12 in children with secretory otitis media: preliminary results of a pilot, uncontrolled study.",
"author": "Di Pierro",
"doi-asserted-by": "publisher",
"first-page": "303",
"journal-title": "Int J Gen Med",
"key": "zoi231183r21",
"volume": "8",
"year": "2015"
},
{
"article-title": "Effect of administration of Streptococcus salivarius K12 on the occurrence of streptococcal pharyngo-tonsillitis, scarlet fever and acute otitis media in 3 years old children.",
"author": "Di Pierro",
"first-page": "4601",
"issue": "21",
"journal-title": "Eur Rev Med Pharmacol Sci",
"key": "zoi231183r22",
"volume": "20",
"year": "2016"
},
{
"DOI": "10.1007/s10096-015-2491-x",
"article-title": "Streptococcus salivarius 24SMB administered by nasal spray for the prevention of acute otitis media in otitis-prone children.",
"author": "Marchisio",
"doi-asserted-by": "publisher",
"first-page": "2377",
"issue": "12",
"journal-title": "Eur J Clin Microbiol Infect Dis",
"key": "zoi231183r23",
"volume": "34",
"year": "2015"
},
{
"DOI": "10.1111/apa.v112.1",
"article-title": "Antibiotic prescriptions during the first 2?years of the COVID-19 pandemic in Finnish children.",
"author": "Kuitunen",
"doi-asserted-by": "publisher",
"first-page": "143",
"issue": "1",
"journal-title": "Acta Paediatr",
"key": "zoi231183r24",
"volume": "112",
"year": "2023"
},
{
"DOI": "10.1016/S0022-3476(88)80380-9",
"article-title": "Rhinovirus in acute otitis media.",
"author": "Arola",
"doi-asserted-by": "publisher",
"first-page": "693",
"issue": "4",
"journal-title": "J Pediatr",
"key": "zoi231183r25",
"volume": "113",
"year": "1988"
},
{
"DOI": "10.1136/bmj-2021-067519",
"article-title": "Indirect effects of the COVID-19 pandemic on childhood infection in England: population based observational study.",
"author": "Kadambari",
"doi-asserted-by": "publisher",
"journal-title": "BMJ",
"key": "zoi231183r26",
"volume": "376",
"year": "2022"
},
{
"DOI": "10.1097/MAO.0000000000003581",
"article-title": "Trends in pediatric acute otitis media burden during the first COVID-19 year.",
"author": "Marom",
"doi-asserted-by": "publisher",
"first-page": "e760",
"issue": "7",
"journal-title": "Otol Neurotol",
"key": "zoi231183r27",
"volume": "43",
"year": "2022"
},
{
"DOI": "10.3389/fcimb.2021.749911",
"article-title": "Otitis media practice during the COVID-19 pandemic.",
"author": "Marom",
"doi-asserted-by": "publisher",
"journal-title": "Front Cell Infect Microbiol",
"key": "zoi231183r28",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.3389/fcimb.2021.768377",
"article-title": "A strong decline in the incidence of childhood otitis media during the COVID-19 pandemic in the Netherlands.",
"author": "Hullegie",
"doi-asserted-by": "publisher",
"journal-title": "Front Cell Infect Microbiol",
"key": "zoi231183r29",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1002/jmv.v93.10",
"article-title": "Rhinovirus spread in children during the COVID-19 pandemic despite social restrictions—a nationwide register study in Finland.",
"author": "Kuitunen",
"doi-asserted-by": "publisher",
"first-page": "6063",
"issue": "10",
"journal-title": "J Med Virol",
"key": "zoi231183r30",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1186/s12245-022-00448-y",
"article-title": "Pneumococcal meningitis secondary to otitis media in two patients with COVID-19 Omicron variant.",
"author": "Franch-Llasat",
"doi-asserted-by": "publisher",
"first-page": "50",
"issue": "1",
"journal-title": "Int J Emerg Med",
"key": "zoi231183r31",
"volume": "15",
"year": "2022"
},
{
"DOI": "10.1016/j.clnu.2007.01.003",
"article-title": "Treatment of acute otitis media with probiotics in otitis-prone children—a double-blind, placebo-controlled randomised study.",
"author": "Hatakka",
"doi-asserted-by": "publisher",
"first-page": "314",
"issue": "3",
"journal-title": "Clin Nutr",
"key": "zoi231183r32",
"volume": "26",
"year": "2007"
},
{
"author": "Armitage",
"key": "zoi231183r20",
"year": "2020"
},
{
"key": "zoi231183r17",
"unstructured": "Kanta services. Accessed February 28, 2023. https://www.kanta.fi/"
},
{
"key": "zoi231183r18",
"unstructured": "Finnish Medical Society Duodecim. Current care guidelines: acute otitis media. September 6, 2017. Accessed September 17, 2022. https://www.kaypahoito.fi/"
}
],
"reference-count": 32,
"references-count": 32,
"relation": {},
"resource": {
"primary": {
"URL": "https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2811238"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [
"A Randomized Clinical Trial"
],
"title": "<i>Streptococcus salivarius</i> Probiotics to Prevent Acute Otitis Media in Children",
"type": "journal-article",
"volume": "6"
}
