An International, Multicenter, Randomized, Double-blind, Adaptive Placebo-controlled Study of the Efficacy and Safety of a Single Administration of Olokizumab and RPH-104 With Standard Therapy in Patients With Severe SARS-CoV-2 Infection (COVID-19)
et al., NCT04380519, NCT04380519, Jan 2022
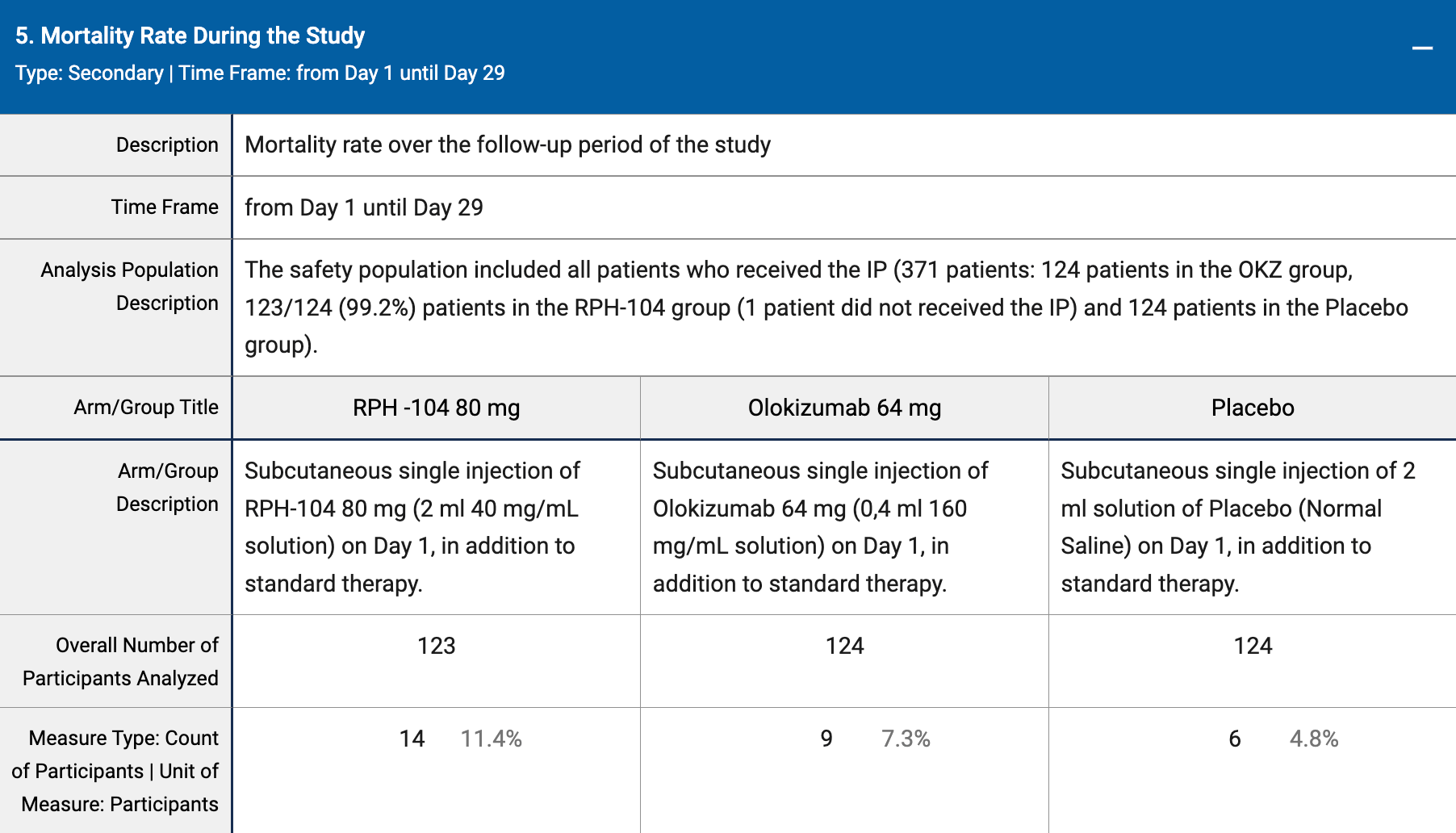
RCT 248 hospitalized patients showing higher mortality (p=0.07) with goflikicept (RPH-104).
Study covers olokizumab and goflikicept.
|
risk of death, 135.2% higher, RR 2.35, p = 0.07, treatment 14 of 123 (11.4%), control 6 of 124 (4.8%), day 29.
|
|
risk of no improvement 2+ points, no change, RR 1.00, p = 1.00, treatment 30 of 124 (24.2%), control 30 of 124 (24.2%), day 29.
|
|
risk of no improvement 1+ points, 2.7% lower, RR 0.97, p = 1.00, treatment 36 of 124 (29.0%), control 37 of 124 (29.8%), NNT 124, day 29.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Samsonov et al., 24 Jan 2022, Double Blind Randomized Controlled Trial, placebo-controlled, Russia, preprint, 1 author, trial NCT04380519 (history).
Contact: sa.grishin@rpharm.ru.