Colchicine and risk of hospitalisation due to COVID-19: a population-based study
et al., Journal of Medical Virology, doi:10.1002/jmv.28496, Jan 2023
Colchicine for COVID-19
5th treatment shown to reduce risk in
September 2020, now with p = 0.0000049 from 54 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
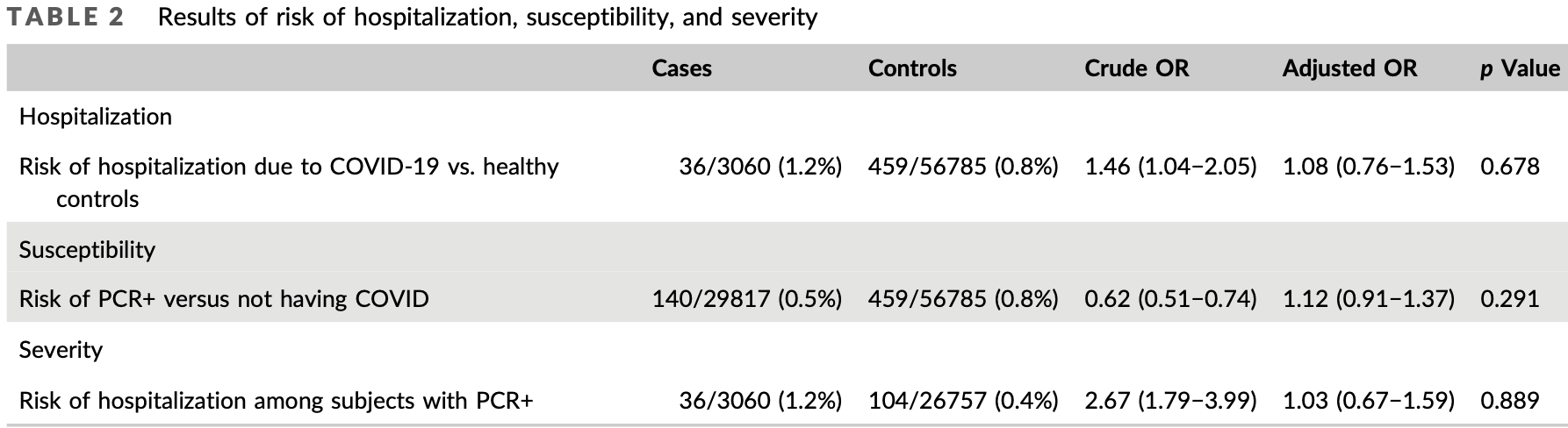
Retrospective 86,652 patients in Spain, showing no significant difference in cases and hospitalization with colchicine use. The different risk for patients prescribed colchicine may not be fully adjusted for. See1.
|
risk of hospitalization, 8.0% higher, OR 1.08, p = 0.68, treatment 36 of 3,060 (1.2%) cases,
459 of 56,785 (0.8%) controls, case control OR.
|
|
risk of case, 12.0% higher, OR 1.12, p = 0.68, treatment 140 of 29,817 (0.5%) cases,
459 of 56,875 (0.8%) controls, NNT 9.0, case control OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Sáenz-Aldea et al., 13 Jan 2023, retrospective, Spain, peer-reviewed, 8 authors.
Contact: angel.salgado.barreira@usc.es.
Colchicine and risk of hospitalization due to COVID‐19: A population‐based study
Journal of Medical Virology, doi:10.1002/jmv.28496
Colchicine is one of the most widely studied and best-known anti-inflammatory treatments. This study aimed to assess the effect of colchicine on risk of hospitalization due to COVID-19; and its effect on susceptibility to and severity of the virus in patients with COVID-19. We carried out a population-based casecontrol study. The following groups were applied: (1) to assess risk of hospitalization, cases were patients with a positive PCR who were hospitalized due to COVID-19, and controls without a positive PCR; (2) to assess susceptibility to COVID-19, cases were patients with a positive PCR (hospitalized and non-hospitalized), and the same controls; (3) to determine potential severity, cases were subjects with COVID-19 hospitalized, and controls patients with COVID-19 nonhospitalised. Different electronic, linked, administrative health and clinical databases were used to extract data on sociodemographic variables, comorbidities, and medications dispensed. The study covered 3060 subjects with a positive PCR who were hospitalized, 26 757 with a positive PCR who were not hospitalized, and 56 785 healthy controls. After adjustment for sociodemographic variables, comorbidities and other treatments, colchicine did not modify risk of hospitalization due to COVID-19 (adjusted odd ratio [OR] 1.08 [95% confidence interval (CI) 0.76−1.53]), patients' susceptibility to contracting the disease (adjusted OR 1.12 (95% CI 0.91−1.37)) or the severity of the infection (adjusted OR 1.03 [95% CI 0.67−1.59]). Our results would neither support the prophylactic use of colchicine for prevention of the infection or hospitalization in any type of patient, nor justify the withdrawal of colchicine treatment due to a higher risk of contracting COVID-19.
00470" project (cofunded by the European Regional Development Fund, "A way to make Europe").
CONFLICT OF INTEREST The authors declare no conflict of interest.
ETHICS STATEMENT The study was approved by the Galician Clinical Research Ethics
References
Ardehali, Colchicine to reduce cardiac injury in COVID-19
Berghezan, Suárez, Tratamientos potenciales para COVID-19 (INFECCIÓN POR SARS-CoV2). Asoc Española Pediatría Atención Primaria Al Cuid la Infanc y la Adolesc
Da, Roura-Piloto, Moral-Escudero, Colchicine in recently hospitalized patients with COVID-19: a randomized controlled trial (COL-COVID), Int J Gen Med
De Abajo, Rodríguez-Martín, Lerma, Use of reninangiotensin-aldosterone system inhibitors and risk of COVID-19 requiring admission to hospital: a case-population study, Lancet
Deftereos, Giannopoulos, Vrachatis, Siasos, Sog, Effect of colchicine vs standard care on cardiac and inflammatory in covid19. The GRECCO-19 Randomized Clinical Trial, JAMA Netw
Deftereos, Siasos, Giannopoulos, The Greek study in the effects of colchicine in COvid-19 complications prevention (GRECCO-19 study): rationale and study design, Hellenic J Cardiol
Diaz, The, Trial, Effects of colchicine on moderate/high-risk hospitalized COVID-19 patients
Dos Santos, Natural history of COVID-19 and current knowledge on treatment therapeutic options, Biomed Pharmacother, doi:10.1016/j.biopha.2020.110493
Díez-Fuertes, Iglesias-Caballero, García-Pérez, A founder effect led early SARS-CoV-2 transmission in Spain, J Virol, doi:10.1128/JVI.01583-20
Elshafei, El-Bardissy, Khalil, Colchicine use might be associated with lower mortality in COVID-19 patients: A metaanalysis, Eur J Clin Invest
Fan, Song, Yip, Zhang, He, Impact of low vaccine coverage on the resurgence of COVID-19 in central and Eastern Europe, One Health, doi:10.1016/j.onehlt.2022.100402
Gendelman, Amital, Bragazzi, Watad, Chodick, Continuous hydroxychloroquine or colchicine therapy does not prevent infection with SARS-CoV-2: insights from a large healthcare database analysis, Autoimmun Rev, doi:10.1016/j.autrev.2020.102566
Golpour, Mousavi, Alimohammadi, The effectiveness of colchicine as an anti-inflammatory drug in the treatment of coronavirus disease 2019: meta-analysis, Int J Immunopathol Pharmacol, doi:10.1177/20587384211031763
Hariyanto, Halim, Jodhinata, Yanto, Kurniawan, Colchicine treatment can improve outcomes of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis, Clin Exp Pharmacol Physiol
Ho, Hu, Lee, The advantages and challenges of using realworld data for patient care, Clin Transl Sci
Huang, Wang, Li, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet
Hyams, Marlow, Maseko, Effectiveness of BNT162b2 and ChAdOx1 nCoV-19 COVID-19 vaccination at preventing hospitalisations in people aged at least 80 years: a test-negative, case-control study, Lancet Infect Dis, doi:10.1016/S1473-3099(21)00330-3
Irony, Case-control studies using "real-world" evidence to assess association, JAMA
Lopes, Bonjorno, Giannini, Beneficial effects of colchicine for moderate to severe COVID-19: A randomised, double-blinded, placebo-controlled clinical trial, RMD Open
Madrid-García, Pérez, Colomer, Influence of colchicine prescription in COVID-19-related hospital admissions: a survival analysis, Ther Adv Musculoskelet Dis
Maggiore, Colchicine counteracting inflammation in COVID-19 pneumonia
Marks, Gruppuso, Adashi, Urgent need for nextgeneration COVID-19 vaccines, JAMA. Published online, doi:10.1001/jama.2022.22759
Mcewan, Robinson, A systematic review of the infectious complications of colchicine and the use of colchicine to treat infections, Semin Arthritis Rheum, doi:10.1016/j.semarthrit.2020.11.007
Mehta, Patel, Chavda, Patel, Efficacy and safety of colchicine in COVID-19: a meta-analysis of randomised controlled trials, RMD Open
Mesa, Hogan, Watson, Modelling the impact of vaccine hesitancy in prolonging the need for non-pharmaceutical interventions to control the COVID-19 pandemic, Commun Med, doi:10.1038/s43856-022-00075-x
Mikolajewska, Fischer, Piechotta, Colchicine for the treatment of COVID-19, Cochrane Database Syst Rev, doi:10.1002/14651858.CD015045
Moore, June, Cytokine release syndrome in severe COVID-19, Science, doi:10.1126/science.abb8925
Nawangsih, Kusmala, Rakhmat, Colchicine and mortality in patients with coronavirus disease 2019 (COVID-19) pneumonia: a systematic review, meta-analysis, and metaregression, Int Immunopharmacol
Olson, Newhams, Halasa, Effectiveness of BNT162b2 vaccine against critical Covid-19 in adolescents, N Engl J Med, doi:10.1056/NEJMoa2117995
Parvathaneni, Gupta, Utilizing drug repurposing against COVID-19 -efficacy, limitations, and challenges, Life Sci
Pelechas, Drossou, Voulgari, Drosos, COVID-19 in patients with gout on colchicine, Rheumatol Int, doi:10.1007/s00296-021-04902-7
Reyes, Hu, Teperman, Anti-inflammatory therapy for COVID-19 infection: the case for colchicine, Ann Rheum Dis
Rothman, Greenland, Lash, Case-Control Studies, doi:10.1002/9780470061596.risk0599
Sattui, Crow, Navarro-Millán, The role of immunomodulatory medications in the treatment of COVID-19, Curr Opin Rheumatol
Siddiqi, Mehra, COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal, J Heart Lung Transplant
Sáenz-Aldea, Salgado-Barreira, Trunk, Colchicine and risk of hospitalization due to COVID-19: a population-based study, J Med Virol
Tardif, Bouabdallaoui, Allier, Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial, Lancet Respir Med
Tenforde, Self, Adams, Association between mRNA vaccination and COVID-19 hospitalization and disease severity, JAMA, doi:10.1001/jama.2021.19499
Tuta-Quintero, Vega-Corredor, Perdomo-Rodríguez, Pimentel, Colchicina, perspectivas de un viejo amigo para la reumatología en la COVID-19: una revisión exploratoria [colchicine
Venkatesan, Repurposing drugs for treatment of COVID-19, Lancet Respir Med, doi:10.1016/S2213-2600(21)00270-8
Veras, Pontelli, Silva, SARS-CoV-2-triggered neutrophil extracellular traps mediate COVID-19 pathology, J Exp Med, doi:10.1084/jem.20201129
Vitiello, Ferrara, Colchicine and SARS-CoV-2: management of the hyperinflammatory state, Respir Med, doi:10.1016/j.rmed.2021.106322
DOI record:
{
"DOI": "10.1002/jmv.28496",
"ISSN": [
"0146-6615",
"1096-9071"
],
"URL": "http://dx.doi.org/10.1002/jmv.28496",
"alternative-id": [
"10.1002/jmv.28496"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2023-01-13"
}
],
"author": [
{
"affiliation": [
{
"name": "Centro de Salud Dávila Santander Spain"
}
],
"family": "Aldea",
"given": "María Sáenz",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0003-4349-4947",
"affiliation": [
{
"name": "Department of Preventive Medicine and Public Health University of Santiago de Compostela Spain"
},
{
"name": "Institute of Health Research of Santiago de Compostela Santiago de Compostela Spain"
}
],
"authenticated-orcid": false,
"family": "Salgado‐Barreira",
"given": "Ángel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Preventive Medicine and Public Health University of Santiago de Compostela Spain"
},
{
"name": "Institute of Health Research of Santiago de Compostela Santiago de Compostela Spain"
},
{
"name": "Consortium for Biomedical Research in Epidemiology & Public Health (CIBER en Epidemiología y Salud Pública‐CIBERESP) University of Santiago de Compostela Santiago de Compostela Spain"
}
],
"family": "Trunk",
"given": "Margarita Taracido",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Consortium for Biomedical Research in Epidemiology & Public Health (CIBER en Epidemiología y Salud Pública‐CIBERESP) University of Santiago de Compostela Santiago de Compostela Spain"
}
],
"family": "Lamas",
"given": "María Piñeiro",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medical Sciences, iBiMED‐Institute of Biomedicine University of Aveiro Aveiro Portugal"
}
],
"family": "Herdeiro",
"given": "Maria T.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Centro de Salud Concepción Arenal Santiago de Compostela, A Coruña Spain"
},
{
"name": "Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV) Santiago de Compostela Spain"
}
],
"family": "Portela‐Romero",
"given": "Manuel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Research Group on Statistics, Econometrics and Health (GRECS) University of Girona Spain"
},
{
"name": "CIBER of Epidemiology and Public Health (CIBERESP) Madrid Spain"
}
],
"family": "Saez",
"given": "Marc",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Preventive Medicine and Public Health University of Santiago de Compostela Spain"
},
{
"name": "Institute of Health Research of Santiago de Compostela Santiago de Compostela Spain"
},
{
"name": "Consortium for Biomedical Research in Epidemiology & Public Health (CIBER en Epidemiología y Salud Pública‐CIBERESP) University of Santiago de Compostela Santiago de Compostela Spain"
}
],
"family": "Figueiras",
"given": "Adolfo",
"sequence": "additional"
}
],
"container-title": "Journal of Medical Virology",
"container-title-short": "Journal of Medical Virology",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2023,
1,
14
]
],
"date-time": "2023-01-14T07:19:41Z",
"timestamp": 1673680781000
},
"deposited": {
"date-parts": [
[
2023,
1,
14
]
],
"date-time": "2023-01-14T07:19:41Z",
"timestamp": 1673680781000
},
"indexed": {
"date-parts": [
[
2023,
1,
15
]
],
"date-time": "2023-01-15T05:09:05Z",
"timestamp": 1673759345406
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
1,
13
]
]
},
"language": "en",
"license": [
{
"URL": "http://onlinelibrary.wiley.com/termsAndConditions#vor",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
1,
13
]
],
"date-time": "2023-01-13T00:00:00Z",
"timestamp": 1673568000000
}
},
{
"URL": "http://doi.wiley.com/10.1002/tdm_license_1.1",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
1,
13
]
],
"date-time": "2023-01-13T00:00:00Z",
"timestamp": 1673568000000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/jmv.28496",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/jmv.28496",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"prefix": "10.1002",
"published": {
"date-parts": [
[
2023,
1,
13
]
]
},
"published-online": {
"date-parts": [
[
2023,
1,
13
]
]
},
"publisher": "Wiley",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1002/jmv.28496"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Virology"
],
"subtitle": [],
"title": "Colchicine and risk of hospitalisation due to COVID‐19: a population‐based study.",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy"
}
