Evaluation of the effect of Loigolactobacillus coryniformis K8 CECT 5711 consumption in health care workers exposed to COVID-19
et al., Frontiers in Nutrition, doi:10.3389/fnut.2022.962566, NCT04366180, Aug 2022
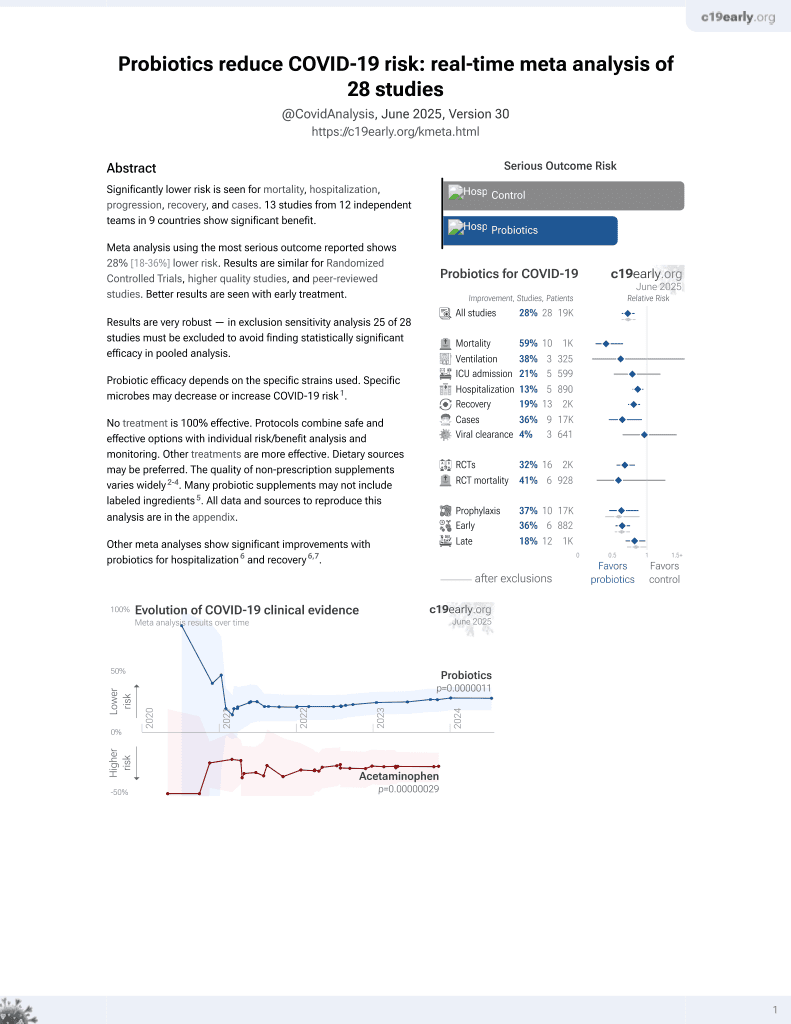
Probiotics for COVID-19
20th treatment shown to reduce risk in
March 2021, now with p = 0.00000044 from 29 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
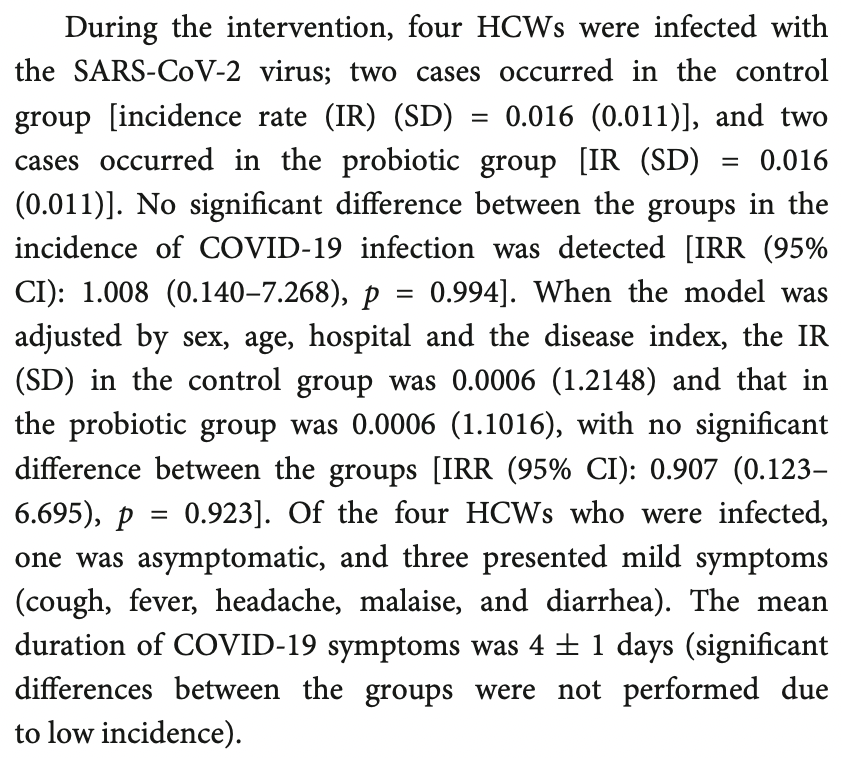
Prophylaxis RCT with 127 probiotics and 128 control healthcare workers in Spain, showing no significant difference in cases. There were only 4 cases. Severity information by arm is not provided. L. coryniformis K8 CECT 5711.
Treatment may help sustain the immune response to vaccination - in the subgroup of subjects for whom more than 81 days had passed since they received the first dose, IgG levels were significantly higher in the treatment group. Patients that started probiotic consumption before the first vaccine dose also reported significantly fewer side effects.
Probiotic efficacy depends on the specific strains used. Specific microbes may decrease or increase COVID-19 risk1.
|
risk of case, 9.3% lower, RR 0.91, p = 0.92, treatment 2 of 127 (1.6%), control 2 of 128 (1.6%), adjusted per study, multivariable.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Rodriguez-Blanque et al., 3 Aug 2022, Double Blind Randomized Controlled Trial, placebo-controlled, Spain, peer-reviewed, 7 authors, study period 24 April, 2020 - 20 July, 2020, trial NCT04366180 (history).
Contact: ruth.blanco@kerry.com.
Evaluation of the effect of Loigolactobacillus coryniformis K8 CECT 5711 consumption in health care workers exposed to COVID-19
Frontiers in Nutrition, doi:10.3389/fnut.2022.962566
In conclusion, the administration of L. coryniformis K8 CECT 5711 to HCWs helps to extend the immune protection generated by the COVID-19 vaccine over time.
Ethics statement The studies involving human participants were reviewed and approved by the Regional Ethical Committee (Granada, Spain). The patients/participants provided their written informed consent to participate in this study.
Author contributions RR-B and MO participated in the conception of the study, designed the methodology, and contributed to the manuscript writing. JS-G participated in the design of the study and critically revised the manuscript. ÁC-V and AA recruited and followed-up the volunteers. JM-L provided study materials and performed the data curation. RB-R participated in the study design, analyzed the data, interpreted the results, and wrote the draft of the manuscript. All authors have read and approved the final manuscript.
Conflict of interest JM-L, MO, and RB-R are workers of Biosearch Life, a Kerry Company, owner of the patent of Loigolactobacillus coryniformis CECT 5711. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material The..
References
Bandyopadhyay, Baticulon, Kadhum, Alser, Ojuka et al., Infection and mortality of healthcare workers worldwide from COVID-19: a systematic review, BMJ Glob Health, doi:10.1136/bmjgh-2020-003097
Chandan, Khan, Deliwala, Mohan, Ramai et al., Significant reduction in vaccine-induced antibody levels and neutralization activity among healthcare workers and nursing home residents 6 months following COVID-19 BNT162b2 MRNA vaccination, Clin Infect Dis, doi:10.1093/cid/ciab963.
Chew, Lee, Tan, Jing, Goh et al., A multinational, multicentre study on the psychological outcomes and associated physical symptoms amongst healthcare workers during COVID-19 outbreak, Brain Behav Immun, doi:10.1016/j.bbi.2020.04.049
Favresse, Bayart, Mullier, Elsen, Eucher et al., Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months, Emerg Microbes Infect
Fernández-Ferreiro, Formigo-Couceiro, Veiga-Gutierrez, Maldonado-Lobón, Hermida-Cao et al., Effects of Loigolactobacillus Coryniformis K8 CECT 5711 on the immune response of elderly subjects to covid-19 vaccination: a randomized controlled trial, Nutrients, doi:10.3390/nu14010228
Fonollá, Gracián, Maldonado-Lobón, Romero, Bédmar et al., Effects of Lactobacillus Coryniformis K8 CECT5711 on the immune response to influenza vaccination and the assessment of common respiratory symptoms in elderly subjects: a randomized controlled trial, Eur J Nutr, doi:10.1007/s00394-017-1573-1
Gilbert, Montefiori, Mcdermott, Fong, Benkeser et al., Surveillance of COVID-19 vaccine effectiveness -a real-time case-control study in Southern Sweden, Epidemiol Infect, doi:10.1017/S0950268822000425
Gómez-Ochoa, Franco, Rojas, Raguindin, Roa-Díaz, None
Kadali, Janagama, Peruru, Gajula, Madathala et al., Non-life-threatening adverse effects with COVID-19 MRNA-1273 vaccine: a randomized cross-sectional study on healthcare workers with detailed selfreported symptoms, J Med Virol, doi:10.1002/jmv.26996
Kurian, Unnikrishnan, Miraj, Bagchi, Banerjee et al., The consumption of two new probiotic strains Lactobacillus Gasseri CECT 5714 and Lactobacillus Coryniformis CECT 5711 boosts the immune system of healthy humans, Int Microbiol, doi:10.1080/19490976.2021.2018899
Lara-Villoslada, Sierra, Boza, Efectos beneficiosos en niños sanos del consumo de un producto lácteo que contiene dos cepas probióticas. Lactobacillus coryniformis CECT5711 y Lactobacillus gasseri CECT5714, Nutr Hosp
Martínez-Cañavate, Sierra, Lara-Villoslada, Romero, Maldonado et al., Probiotic dairy product containing L. Gasseri CECT5714 and L. Coryniformis CECT5711 induces immunological changes in children suffering from allergy, Pediatr Allergy Immunol, doi:10.1186/s12986-016-0154-2
Pormohammad, Zarei, Ghorbani, Mohammadi, Razizadeh, None
Sahu, Amrithanand, Mathew, Aggarwal, Nayer et al., COVID-19 in health care workers -a systematic review and meta-analysis, Am J Emerg Med, doi:10.1016/j.ajem.2020.05.113
Salvagno, Henry, Pighi, Nitto, Gianfilippi et al., Threemonth analysis of total humoral response to pfizer BNT162b2 MRNA COVID-19 vaccination in healthcare workers, J Infect, doi:10.1016/j.jinf.2021.06.024
Soriano, De Mendoza, Gómez-Gallego, Corral, Barreiro, Third Wave of COVID-19 in Madrid Spain, Int J Infect Dis, doi:10.1016/j.ijid.2021.04.074
Turner, Efficacy and safety of COVID-19 vaccines: a systematic review and meta-analysis of randomized clinical trials, Vaccines, doi:10.3390/vaccines9050467
Who, Coronavirus (COVID-19) Dashboard
Wyssmann, COVID-19 in healthcare workers: a living systematic review and meta-analysis of prevalence risk factors clinical characteristics and outcomes, Am J Epidemiol, doi:10.1093/aje/kwaa191
Zee, Lai, Ho, Leung, Fung et al., Serological response to MRNA and inactivated COVID-19 vaccine in healthcare workers in Hong Kong: decline in antibodies 12 weeks after two doses, Hong Kong Med J, doi:10.12809/hkmj219744
DOI record:
{
"DOI": "10.3389/fnut.2022.962566",
"ISSN": [
"2296-861X"
],
"URL": "http://dx.doi.org/10.3389/fnut.2022.962566",
"abstract": "<jats:p>Following the spread of the SARS-CoV-2 coronavirus, an unprecedented burden has been placed on health care systems, with health care workers (HCWs) being most at risk of COVID-19 infection. The effect of the probiotic <jats:italic>Loigolactobacillus coryniformis</jats:italic> K8 CECT 5711 on frontline HCWs exposed to the virus was studied in a randomized, double-blind, placebo controlled trial. Parameters related to the incidence and severity of COVID-19 as well as the immune response and the side effects of the COVID-19 vaccine were evaluated. For 2 months, a group of 250 front-line HCWs over the age of 20 was randomly allocated to receive either <jats:italic>L. coryniformis</jats:italic> K8 or a placebo daily. SARS-CoV-2 infection incidence was verified via PCR or antigen test. In those volunteers who were vaccinated during the intervention, serum levels of specific IgG were analyzed at the end of the study. The incidence of COVID-19 infection was very low [IR (SD) = 0.016 (0.011)], and no significant difference was found between the groups [IRR (95% CI): 1.008 (0.140–7.268), <jats:italic>p</jats:italic> = 0.994]. For immune response analysis, the total sample was divided according to the days between the first dose and the antibody analysis (cutoff points were set at ≤ 56, 57–80 and ≥ 81 days). The specific IgG level decreased over time (<jats:italic>p</jats:italic> &gt; 0.001). However, in the subgroup of subjects for whom more than 81 days had passed since they received the first dose, the specific IgG levels were significantly higher in the those that took the <jats:italic>L. coryniformis</jats:italic> K8 [7.12 (0.21)] than in the control group [6.48 (0.19)] (<jats:italic>P</jats:italic> = 0.040). Interestingly, the subjects who started probiotic consumption before the first dose reported significantly fewer side effects (of any kind) at the 1st dose of the vaccine (OR: 0.524, <jats:italic>p</jats:italic> = 0.043), specifically less arm pain (OR: 0.467, <jats:italic>p</jats:italic> = 0.017). In conclusion, the administration of <jats:italic>L. coryniformis</jats:italic> K8 CECT 5711 to HCWs helps to extend the immune protection generated by the COVID-19 vaccine over time.</jats:p>",
"alternative-id": [
"10.3389/fnut.2022.962566"
],
"author": [
{
"affiliation": [],
"family": "Rodriguez-Blanque",
"given": "Raquel",
"sequence": "first"
},
{
"affiliation": [],
"family": "Sánchez-García",
"given": "Juan Carlos",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cobos-Vargas",
"given": "Ángel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aguilar Quesada",
"given": "Ana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Maldonado-Lobón",
"given": "Jose A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Olivares",
"given": "Mónica",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Blanco-Rojo",
"given": "Ruth",
"sequence": "additional"
}
],
"container-title": "Frontiers in Nutrition",
"container-title-short": "Front. Nutr.",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2022,
8,
3
]
],
"date-time": "2022-08-03T04:25:12Z",
"timestamp": 1659500712000
},
"deposited": {
"date-parts": [
[
2022,
8,
3
]
],
"date-time": "2022-08-03T04:25:19Z",
"timestamp": 1659500719000
},
"funder": [
{
"DOI": "10.13039/501100011011",
"doi-asserted-by": "publisher",
"name": "Junta de Andalucía"
}
],
"indexed": {
"date-parts": [
[
2022,
8,
3
]
],
"date-time": "2022-08-03T04:44:12Z",
"timestamp": 1659501852923
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
8,
3
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
8,
3
]
],
"date-time": "2022-08-03T00:00:00Z",
"timestamp": 1659484800000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fnut.2022.962566/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2022,
8,
3
]
]
},
"published-online": {
"date-parts": [
[
2022,
8,
3
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"journal-title": "Coronavirus (COVID-19) Dashboard.",
"key": "B1",
"year": "2020"
},
{
"DOI": "10.1016/j.bbi.2020.04.049",
"article-title": "A multinational, multicentre study on the psychological outcomes and associated physical symptoms amongst healthcare workers during COVID-19 outbreak.",
"author": "Chew",
"doi-asserted-by": "publisher",
"first-page": "559",
"journal-title": "Brain Behav Immun.",
"key": "B2",
"volume": "88",
"year": "2020"
},
{
"DOI": "10.1093/aje/kwaa191",
"article-title": "COVID-19 in healthcare workers: a living systematic review and meta-analysis of prevalence risk factors clinical characteristics and outcomes.",
"author": "Gómez-Ochoa",
"doi-asserted-by": "publisher",
"first-page": "161",
"journal-title": "Am J Epidemiol.",
"key": "B3",
"volume": "190",
"year": "2020"
},
{
"DOI": "10.1016/j.ajem.2020.05.113",
"article-title": "COVID-19 in health care workers – a systematic review and meta-analysis.",
"author": "Sahu",
"doi-asserted-by": "publisher",
"first-page": "1727",
"journal-title": "Am J Emerg Med.",
"key": "B4",
"volume": "38",
"year": "2020"
},
{
"DOI": "10.1016/j.arcmed.2021.03.002",
"article-title": "Probiotics in prevention and treatment of covid-19: current perspective and future prospects.",
"author": "Kurian",
"doi-asserted-by": "publisher",
"first-page": "582",
"journal-title": "Arch Med Res.",
"key": "B5",
"volume": "52",
"year": "2021"
},
{
"DOI": "10.1016/j.nutres.2020.12.014",
"article-title": "Probiotics: a potential immunomodulator in COVID-19 infection management.",
"author": "Singh",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Nutr Res.",
"key": "B6",
"volume": "87",
"year": "2021"
},
{
"DOI": "10.1016/j.micpath.2020.104452",
"article-title": "The immunomodulatory effects of probiotics on respiratory viral infections: a hint for COVID-19 treatment?",
"author": "Mahooti",
"doi-asserted-by": "publisher",
"journal-title": "Microb Pathog.",
"key": "B7",
"volume": "148",
"year": "2020"
},
{
"DOI": "10.1080/19490976.2021.2018899",
"article-title": "Probiotic improves symptomatic and viral clearance in Covid19 outpatients: a randomized, quadruple-blinded, placebo-controlled trial.",
"author": "Gutiérrez-Castrellón",
"doi-asserted-by": "publisher",
"journal-title": "Gut Microbes.",
"key": "B8",
"volume": "14",
"year": "2022"
},
{
"article-title": "The consumption of two new probiotic strains Lactobacillus Gasseri CECT 5714 and Lactobacillus Coryniformis CECT 5711 boosts the immune system of healthy humans.",
"author": "Olivares",
"first-page": "47",
"journal-title": "Int Microbiol.",
"key": "B9",
"volume": "9",
"year": "2006"
},
{
"article-title": "Efectos beneficiosos en niños sanos del consumo de un producto lácteo que contiene dos cepas probióticas. Lactobacillus coryniformis CECT5711 y Lactobacillus gasseri CECT5714.",
"author": "Lara-Villoslada",
"first-page": "496",
"journal-title": "Nutr Hosp.",
"key": "B10",
"volume": "22",
"year": "2007"
},
{
"DOI": "10.1111/j.1399-3038.2008.00833.x",
"article-title": "Probiotic dairy product containing L. Gasseri CECT5714 and L. Coryniformis CECT5711 induces immunological changes in children suffering from allergy.",
"author": "Martínez-Cañavate",
"doi-asserted-by": "publisher",
"first-page": "592",
"journal-title": "Pediatr Allergy Immunol.",
"key": "B11",
"volume": "20",
"year": "2009"
},
{
"DOI": "10.1186/s12986-016-0154-2",
"article-title": "Evaluation of Lactobacillus Coryniformis CECT5711 strain as a coadjuvant in a vaccination process: a randomised clinical trial in healthy adults.",
"author": "Redondo",
"doi-asserted-by": "publisher",
"journal-title": "Nutr Metab.",
"key": "B12",
"volume": "14",
"year": "2017"
},
{
"DOI": "10.1007/s00394-017-1573-1",
"article-title": "Effects of Lactobacillus Coryniformis K8 CECT5711 on the immune response to influenza vaccination and the assessment of common respiratory symptoms in elderly subjects: a randomized controlled trial.",
"author": "Fonollá",
"doi-asserted-by": "publisher",
"first-page": "83",
"journal-title": "Eur J Nutr.",
"key": "B13",
"volume": "58",
"year": "2019"
},
{
"DOI": "10.3390/nu14010228",
"article-title": "Effects of Loigolactobacillus Coryniformis K8 CECT 5711 on the immune response of elderly subjects to covid-19 vaccination: a randomized controlled trial.",
"author": "Fernández-Ferreiro",
"doi-asserted-by": "publisher",
"journal-title": "Nutrients.",
"key": "B14",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1136/bmjgh-2020-003097",
"article-title": "Infection and mortality of healthcare workers worldwide from COVID-19: a systematic review.",
"author": "Bandyopadhyay",
"doi-asserted-by": "publisher",
"journal-title": "BMJ Glob Health.",
"key": "B15",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1002/jmv.27457",
"article-title": "Postvaccination SARS-CoV-2 infection among healthcare workers: a systematic review and meta-analysis.",
"author": "Chandan",
"doi-asserted-by": "publisher",
"first-page": "1428",
"journal-title": "J Med Virol.",
"key": "B16",
"volume": "94",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciab963.",
"article-title": "Significant reduction in vaccine-induced antibody levels and neutralization activity among healthcare workers and nursing home residents 6 months following COVID-19 BNT162b2 MRNA vaccination.",
"author": "Canaday",
"doi-asserted-by": "publisher",
"journal-title": "Clin Infect Dis.",
"key": "B17",
"year": "2021"
},
{
"DOI": "10.1016/j.jinf.2021.06.024",
"article-title": "Three-month analysis of total humoral response to pfizer BNT162b2 MRNA COVID-19 vaccination in healthcare workers.",
"author": "Salvagno",
"doi-asserted-by": "publisher",
"first-page": "e4",
"journal-title": "J Infect.",
"key": "B18",
"volume": "83",
"year": "2021"
},
{
"journal-title": "COVID-19 en España.",
"key": "B19",
"year": "2022"
},
{
"DOI": "10.1016/j.ijid.2021.04.074",
"article-title": "Third Wave of COVID-19 in Madrid Spain.",
"author": "Soriano",
"doi-asserted-by": "publisher",
"first-page": "212",
"journal-title": "Int J Infect Dis.",
"key": "B20",
"volume": "107",
"year": "2021"
},
{
"DOI": "10.12809/hkmj219744",
"article-title": "Serological response to MRNA and inactivated COVID-19 vaccine in healthcare workers in Hong Kong: decline in antibodies 12 weeks after two doses.",
"author": "Zee",
"doi-asserted-by": "publisher",
"first-page": "380",
"journal-title": "Hong Kong Med J.",
"key": "B21",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1080/22221751.2021.1953403",
"article-title": "Antibody titres decline 3-month post-vaccination with BNT162b2.",
"author": "Favresse",
"doi-asserted-by": "publisher",
"first-page": "1495",
"journal-title": "Emerg Microbes Infect.",
"key": "B22",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2114583",
"article-title": "Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months.",
"author": "Levin",
"doi-asserted-by": "publisher",
"journal-title": "New Engl J Med.",
"key": "B23",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1126/science.abm3425",
"article-title": "Immune correlates analysis of the MRNA-1273 COVID-19 vaccine efficacy clinical trial.",
"author": "Gilbert",
"doi-asserted-by": "publisher",
"first-page": "43",
"journal-title": "Science.",
"key": "B24",
"volume": "375",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2109072",
"article-title": "Covid-19 breakthrough infections in vaccinated health care workers.",
"author": "Bergwerk",
"doi-asserted-by": "publisher",
"first-page": "1474",
"journal-title": "N Engl J Med.",
"key": "B25",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1017/S0950268822000425",
"article-title": "Surveillance of COVID-19 vaccine effectiveness - a real-time case-control study in Southern Sweden.",
"author": "Björk",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Epidemiol Infect.",
"key": "B26",
"volume": "150",
"year": "2022"
},
{
"DOI": "10.3390/vaccines9050467",
"article-title": "Efficacy and safety of COVID-19 vaccines: a systematic review and meta-analysis of randomized clinical trials.",
"author": "Pormohammad",
"doi-asserted-by": "publisher",
"journal-title": "Vaccines.",
"key": "B27",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1002/jmv.26996",
"article-title": "Non-life-threatening adverse effects with COVID-19 MRNA-1273 vaccine: a randomized cross-sectional study on healthcare workers with detailed self-reported symptoms.",
"author": "Kadali",
"doi-asserted-by": "publisher",
"first-page": "4420",
"journal-title": "J Med Virol.",
"key": "B28",
"volume": "93",
"year": "2021"
}
],
"reference-count": 28,
"references-count": 28,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.frontiersin.org/articles/10.3389/fnut.2022.962566/full"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Nutrition and Dietetics",
"Endocrinology, Diabetes and Metabolism",
"Food Science"
],
"subtitle": [],
"title": "Evaluation of the effect of Loigolactobacillus coryniformis K8 CECT 5711 consumption in health care workers exposed to COVID-19",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.3389/crossmark-policy",
"volume": "9"
}