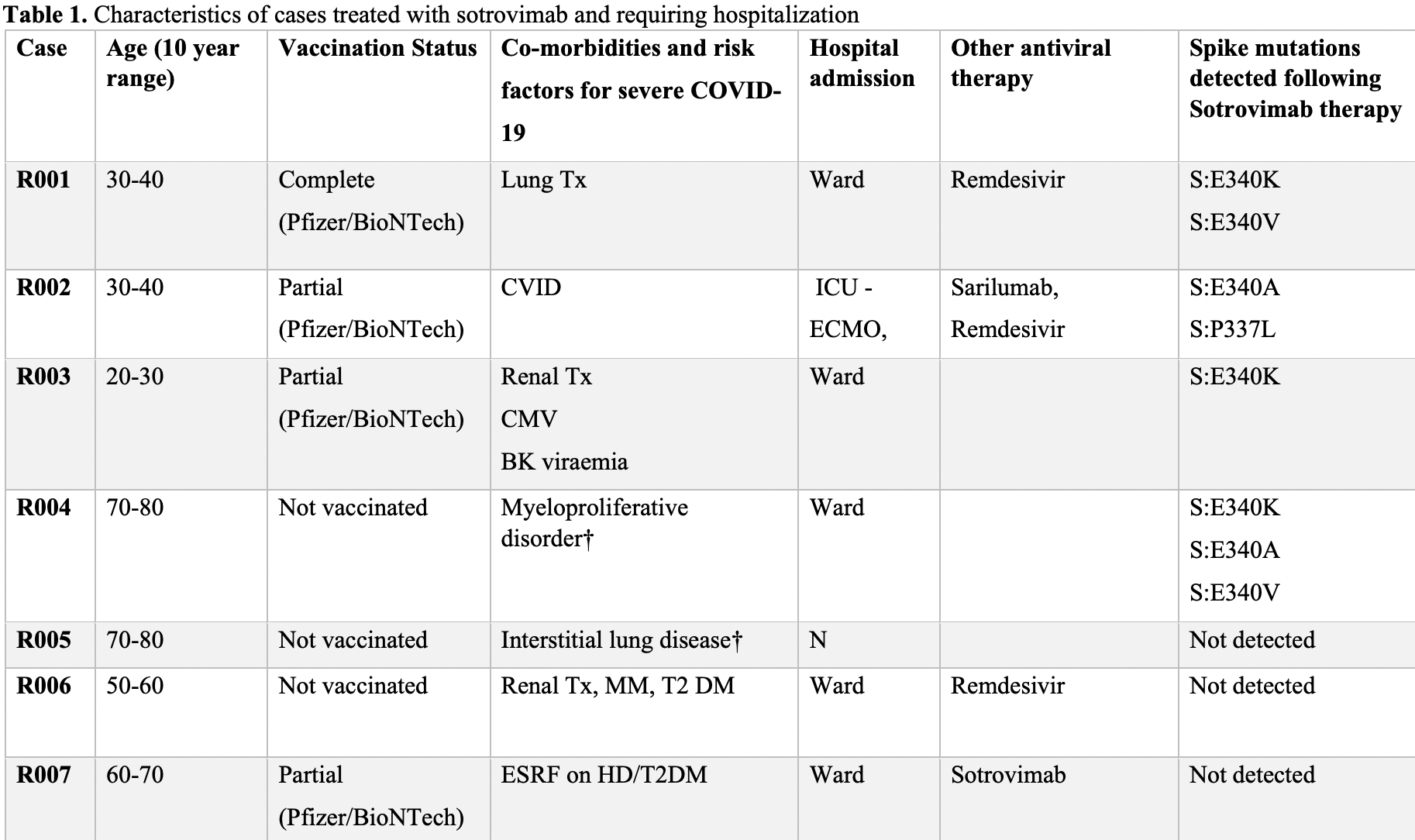
Resistance conferring mutations in SARS-CoV-2 delta following sotrovimab infusion
et al., medRxiv, doi:10.1101/2021.12.18.21267628, Dec 2021
Sotrovimab for COVID-19
45th treatment shown to reduce risk in
August 2022, now with p = 0.00048 from 29 studies, recognized in 42 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 100 sotrovimab patients in Australia, 23 PCR+ more than 10 days post-infusion (68 with status unknown), showing rapid development of spike gene mutations that have been shown to confer high level resistance to sotrovimab in vitro.
Efficacy is variant dependent. In Vitro studies predict lower efficacy for BA.11-3, BA.4, BA.54, XBB.1.9.3, XBB.1.5.24, XBB.2.9, CH.1.15, and no efficacy for BA.26, XBB, XBB.1.5, ХВВ.1.9.17, XBB.1.16, BQ.1.1.45, and CL.15. US EUA has been revoked.
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
4.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
5.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
Rockett et al., 21 Dec 2021, preprint, 26 authors.
Abstract: medRxiv preprint doi: https://doi.org/10.1101/2021.12.18.21267628; this version posted December 21, 2021. The copyright holder for this
preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in
perpetuity.
It is made available under a CC-BY-NC-ND 4.0 International license .
RESISTANCE CONFERRING MUTATIONS IN SARS-CoV-2 DELTA FOLLOWING
SOTROVIMAB INFUSION
Rebecca J Rockett1,4*, Kerri Basile2, Susan Maddocks2,3, Winkie Fong1, Jessica E Agius1,
Jessica Johnson Mackinnon1,4, Alicia Arnott1,2, Shona Chandra1, Mailie Gall1,2, Jenny
Draper1,2, Elena Martinez1,2, Eby M Sim1,2, Clement Lee2, Christine Ngo2, Marc
Ramsperger2, Andrew N Ginn2,4, Qinning Wang1,2, Michael Fennell2, Danny Ko2, H Ling
Lim2,5, Nicky Gilroy3, Matthew V N O’Sullivan1,2,3,4, Sharon C-A Chen1,2,4, Jen Kok1,2,
Dominic E Dwyer1,2,4, Vitali Sintchenko1,2,4*
1
Centre for Infectious Diseases and Microbiology-Public Health, Westmead Hospital,
Westmead, New South Wales, Australia
2
Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales
Health Pathology, Institute for Clinical Pathology and Medical Research, Westmead, New
South Wales, Australia
3
Department of Infectious Diseases, Westmead Hospital, Western Sydney Local Health
District, Sydney, New South Wales, Australia
4
Sydney Institute of Infectious Diseases, University of Sydney, Sydney, New South Wales,
Australia
5
Parramatta Public Health Unit, Western Sydney Local Health District, Parramatta, New South
Wales, Australia
*Corresponding authors
Dr Rebecca J. Rockett and Prof Vitali Sintchenko
rebecca.rockett@health.nsw.gov.au and Vitali.Sintchenko@health.nsw.gov.au
Sydney Institute of Infectious Diseases, University of Sydney, Sydney, New South Wales,
Australia
NOTE: This preprint reports new research that has not been certified by peer review and should not be used to guide clinical practice.
medRxiv preprint doi: https://doi.org/10.1101/2021.12.18.21267628; this version posted December 21, 2021. The copyright holder for this
preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in
perpetuity.
It is made available under a CC-BY-NC-ND 4.0 International license .
ABSTRACT (100 words)
Several Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) neutralising
monoclonal antibodies (mAbs) have received emergency use authorisation by regulatory
agencies for treatment and prevention of Coronavirus Disease 2019 (COVID-19), including in
patients at risk for progression to severe disease. Here we report the persistence of viable
SARS-CoV-2 in patients treated with sotrovimab and the rapid development of spike gene
mutations that have been shown to confer high level resistance to sotrovimab in vitro. We
highlight the need for SARS-CoV-2 genomic surveillance in at risk individuals to inform
stewardship of mAbs use and prevent potential treatment failures.
medRxiv preprint doi: https://doi.org/10.1101/2021.12.18.21267628; this version posted December 21, 2021. The copyright holder for this
preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in
perpetuity.
It is made available under a CC-BY-NC-ND 4.0 International license .
DOI record:
{
"DOI": "10.1101/2021.12.18.21267628",
"URL": "http://dx.doi.org/10.1101/2021.12.18.21267628",
"abstract": "<jats:p>Several Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) neutralising monoclonal antibodies (mAbs) have received emergency use authorisation by regulatory agencies for treatment and prevention of Coronavirus Disease 2019 (COVID-19), including in patients at risk for progression to severe disease. Here we report the persistence of viable SARS-CoV-2 in patients treated with sotrovimab and the rapid development of spike gene mutations that have been shown to confer high level resistance to sotrovimab in vitro. We highlight the need for SARS-CoV-2 genomic surveillance in at risk individuals to inform stewardship of mAbs use and prevent potential treatment failures.</jats:p>",
"accepted": {
"date-parts": [
[
2021,
12,
21
]
]
},
"author": [
{
"ORCID": "http://orcid.org/0000-0003-4329-5198",
"affiliation": [],
"authenticated-orcid": false,
"family": "Rockett",
"given": "Rebecca J",
"sequence": "first"
},
{
"affiliation": [],
"family": "Basile",
"given": "Kerri",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Maddocks",
"given": "Susan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fong",
"given": "Winkie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Agius",
"given": "Jessica E",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Johnson-Mackinnon",
"given": "Jessica",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Arnott",
"given": "Alicia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chandra",
"given": "Shona",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gall",
"given": "Mailie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Draper",
"given": "Jenny L",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Martinez",
"given": "Elena",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sim",
"given": "Eby M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lee",
"given": "Clement",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ngo",
"given": "Christine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ramsperger",
"given": "Marc",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ginn",
"given": "Andrew N",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Qinning",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fennell",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ko",
"given": "Danny",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lim",
"given": "Ling",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gilroy",
"given": "Nicky",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sullivan",
"given": "Matthew VN",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chen",
"given": "Sharon C-A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kok",
"given": "Jen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dwyer",
"given": "Dominic E",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sintchenko",
"given": "Vitali L",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
12,
22
]
],
"date-time": "2021-12-22T03:15:16Z",
"timestamp": 1640142916000
},
"deposited": {
"date-parts": [
[
2021,
12,
22
]
],
"date-time": "2021-12-22T03:15:17Z",
"timestamp": 1640142917000
},
"group-title": "Infectious Diseases (except HIV/AIDS)",
"indexed": {
"date-parts": [
[
2021,
12,
22
]
],
"date-time": "2021-12-22T06:51:50Z",
"timestamp": 1640155910022
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2021,
12,
21
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2021.12.18.21267628",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2021,
12,
21
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2021,
12,
21
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference-count": 0,
"references-count": 0,
"relation": {},
"score": 1,
"short-container-title": [],
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": [
"RESISTANCE CONFERRING MUTATIONS IN SARS-CoV-2 DELTA FOLLOWING SOTROVIMAB INFUSION"
],
"type": "posted-content"
}
