A Randomized Controlled Trial of the Efficacy of Systemic Enzymes and Probiotics in the Resolution of Post-COVID Fatigue
et al., Medicines, doi:10.3390/medicines8090047, CTRI/2021/05/033576, Aug 2021
Probiotics for COVID-19
20th treatment shown to reduce risk in
March 2021, now with p = 0.00000044 from 29 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
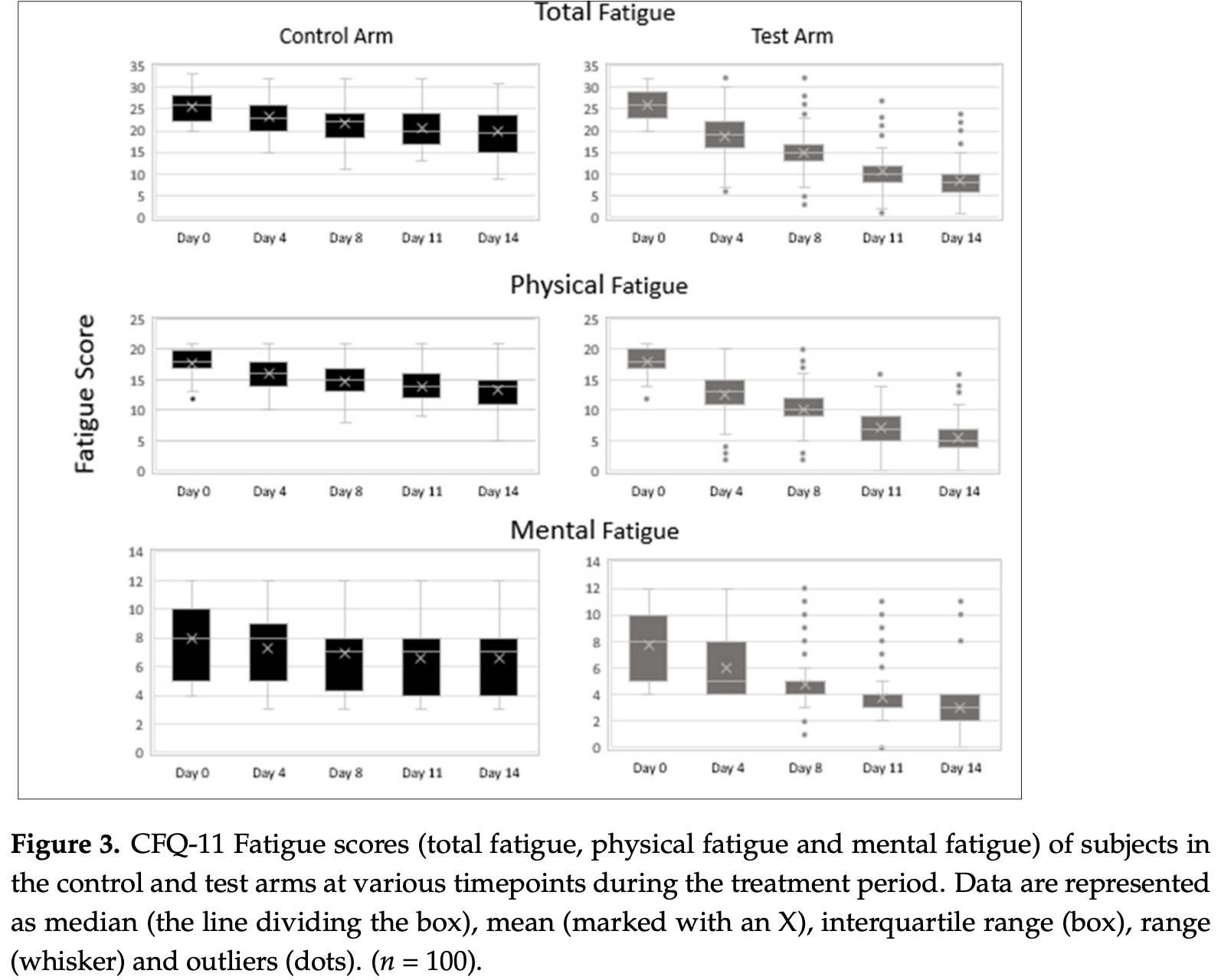
RCT 200 long-COVID patients in India, 100 treated with ImmunoSEB and ProbioSEB CSC3, showing improved recovery of post-COVID-19 fatigue with treatment. CTRI/2021/05/033576.
Probiotic efficacy depends on the specific strains used. Specific microbes may decrease or increase COVID-19 risk1.
|
risk of no recovery, 89.4% lower, RR 0.11, p < 0.001, treatment 9 of 100 (9.0%), control 85 of 100 (85.0%), NNT 1.3, day 14, fatigue.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Rathi et al., 30 Aug 2021, Double Blind Randomized Controlled Trial, placebo-controlled, India, peer-reviewed, 3 authors, study period 12 May, 2021 - 31 May, 2021, trial CTRI/2021/05/033576.
Contact: akrathi@advancedenzymes.com (corresponding author), swati@advancedenzymes.com, drnehashah772@gmail.com.
A Randomized Controlled Trial of the Efficacy of Systemic Enzymes and Probiotics in the Resolution of Post-COVID Fatigue
Medicines, doi:10.3390/medicines8090047
Muscle fatigue and cognitive disturbances persist in patients after recovery from acute COVID-19 disease. However, there are no specific treatments for post-COVID fatigue. Objective: To evaluate the efficacy and safety of the health supplements ImmunoSEB (systemic enzyme complex) and ProbioSEB CSC3 (probiotic complex) in patients suffering from COVID-19 induced fatigue. A randomized, multicentric, double blind, placebo-controlled trial was conducted in 200 patients with a complaint of post-COVID fatigue. The test arm (n = 100) received the oral supplements for 14 days and the control arm (n = 100) received a placebo. Treatment efficacy was compared using the Chalder Fatigue scale (CFQ-11), at various time points from days 1 to 14. The supplemental treatment resulted in resolution of fatigue in a greater percentage of subjects in the test vs. the control arm (91% vs. 15%) on day 14. Subjects in the test arm showed a significantly greater reduction in total as well as physical and mental fatigue scores at all time points vs. the control arm. The supplements were well tolerated with no adverse events reported. This study demonstrates that a 14 days supplementation of ImmunoSEB + ProbioSEB CSC3 resolves post-COVID-19 fatigue and can improve patients' functional status and quality of life.
Institutional Review Board Statement: The present clinical trial was conducted as per the ethical principles contained in the current revision of the "Declaration of Helsinki 2013", ICH harmonized guideline integrated addendum to ICH E6(R1): Guidelines for Good Clinical Practice ICH E6(R2) and following the "Ethical Guidelines for Biomedical Research on Human Subjects" issued by the Indian Council of Medical Research and all other applicable laws and regulations of the country. Informed Consent Statement: Informed written consent was obtained from all participants. No vulnerable subject participated in the study.
Conflicts of Interest: A.R. and S.J. are paid employees of Advanced Enzyme Technologies, which sponsored the study and has a corporate affiliation with Specialty Enzymes and Probiotics. Specialty Enzymes and Probiotics had no role in the study design and actual conduct of the study. N.S. has no conflict of interest to report.
References
Ando, Mimura, Johansson, Hanson, Mougiakakos et al., Transduction with the antioxidant enzyme catalase protects human T cells against oxidative stress, J. Immunol, doi:10.4049/jimmunol.181.12.8382
Azad, Sarker, Wan, Immunomodulatory effects of probiotics on cytokine profiles, Biomed. Res. Int, doi:10.1155/2018/8063647
Butler, Chalder, Ron, Wessely, Cognitive behaviour therapy in chronic fatigue syndrome, J. Neurol. Neurosurg. Psychiatry, doi:10.1136/jnnp.54.2.153
Carabotti, Scirocco, Maselli, Severi, The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems, Ann. Gastroenterol
Chalder, Berelowitz, Pawlikowska, Watts, Wessely et al., Development of a fatigue scale, J. Psychosom. Res, doi:10.1016/0022-3999(93)90081-P
Chitnis, Rathi, In-vitro virucidal activity of commercial enzyme preparations against SARS-CoV-2 virus, doi:10.20944/preprints202012.0543.v1
Eguchi, Fujitani, Nakagawa, Prevention of respiratory syncytial virus infection with probiotic lactic acid bacterium Lactobacillus gasseri SBT2055, Sci. Rep, doi:10.1038/s41598-019-39602-7
Farhadi, Bracho-Sanchez, Freeman, Keselowsky, Hudalla, Enzymes as immunotherapeutics, Bioconjugate Chem, doi:10.1021/acs.bioconjchem.7b00719
Gaspani, Limiroli, Ferrario, Bianchi, In vivo and in vitro effects of bromelain on PGE2 and SP concentrations in the inflammatory exudate in rats, Pharmacology, doi:10.1159/000056191
Gerwyn, Maes, Mechanisms explaining muscle fatigue and muscle pain in patients with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): A Review of recent findings, Curr. Rheumatol. Rep, doi:10.1007/s11926-017-0628-x
Gupta, Vi, Sharma, Tirkey, Rishi et al., Curcumin, a polyphenolic antioxidant, attenuates chronic fatigue syndrome in murine water immersion stress model, Immunobiology, doi:10.1016/j.imbio.2008.04.003
Jackson, The Chalder fatigue scale (CFQ 11), Occup. Med, doi:10.1093/occmed/kqu168
Jackson, The general health questionnaire, Occup. Med, doi:10.1093/occmed/kql169
Jadhav, Shah, Rathi, Rathi, Rathi, Serratiopeptidase: Insights into the therapeutic applications, Biotechnol. Rep, doi:10.1016/j.btre.2020.e00544
Kedor, Freitag, Meyer-Arndt, Wittke, Steinbeis et al., Chronic COVID-19 syndrome and chronic fatigue syndrome (ME/CFS) following the first pandemic wave in Germany-A first analysis of a prospective observational study, medRxiv, doi:10.1101/2021.02.06.21249256
Kim, Yun, Oh, Choi, Mind-altering with the gut: Modulation of the gut-brain axis with probiotics, J. Microbiol, doi:10.1007/s12275-018-8032-4
Lee, Shin, Park, Kim, Lee et al., Depression as a mediator of chronic fatigue and post-traumatic stress symptoms in middle east respiratory syndrome survivors, Psychiatry. Investig, doi:10.30773/pi.2018.10.22.3
Liu, Zheng, Cao, Ren, Zhu et al., Amelioration of oxidant stress by the defensin lysozyme, Am. J. Physiol. Endocrinol. Metab, doi:10.1152/ajpendo.00349.2005
Loge, Ekeberg, Kaasa, Fatigue in the general Norwegian population: Normative data and associations, J. Psychosom. Res, doi:10.1016/S0022-3999(97)00291-2
Maccarone, Magro, Tognolo, Masiero, Post COVID-19 persistent fatigue: A proposal for rehabilitative interventions in the spa setting, Intern. J. Biometeorol
Mahase, Covid-19: What do we know about "long covid, BMJ, doi:10.1136/bmj.m2815
Malaczewska, Kaczorek-Lukowska, Wojcik, Siwicki, Antiviral effects of nisin, lysozyme, lactoferrin and their mixtures against bovine viral diarrhoea virus, BMC Veter. Res, doi:10.1186/s12917-019-2067-6
Marotta, Sarno, Del Casale, Pane, Mogna et al., Effects of probiotics on cognitive reactivity, mood, and sleep quality, Front. Psychiatry, doi:10.3389/fpsyt.2019.00164
Marshall, The lasting misery of coronavirus long-haulers, Nature, doi:10.1038/d41586-020-02598-6
Morriss, Wearden, Mullis, Exploring the validity of the Chalder Fatigue scale in chronic fatigue syndrome, J. Psychosom. Res, doi:10.1016/S0022-3999(98)00022-1
Morshedi, Hashemi, Moazzen, Immunomodulatory and anti-inflammatory effects of probiotics in multiple sclerosis: A systematic review, J. Neuroinflam, doi:10.1186/s12974-019-1611-4
Murakami, Sasaki, Sugahara, Lysozyme stimulates immunoglobulin production by human-human hybridoma and human peripheral blood lymphocytes, Cytotechnology, doi:10.1023/A:1007936629501
Ng, Peters, Ho, Lim, Yeo, A meta-analysis of the use of probiotics to alleviate depressive symptoms, J. Affect. Disord, doi:10.1016/j.jad.2017.11.063
Nipate, Tiwari, Antioxidant and immunomodulatory properties of Spilanthes oleracea with potential effect in chronic fatigue syndrome infirmity, J. Ayurv. Int. Med, doi:10.1016/j.jaim.2017.08.008
O'connor, COVID-19 Fatigue, JACC Heart Fail, doi:10.1016/j.jchf.2020.06.001
O'sullivan, Long-term sequelae following previous coronavirus epidemics, Clin. Med, doi:10.7861/clinmed.2020-0204
Ons Website, None
Parate, Shah, Management of post COVID-19 fatigue using systemic enzymes and probiotics-Case series, Med. J. Clin. Trials Case Stud
Perrin, Riste, Hann, Into the looking glass: Post-viral syndrome post COVID-19, Med. Hypotheses, doi:10.1016/j.mehy.2020.110055
Poenaru, Abdallah, Corrales-Medina, Cowan, COVID-19 and post-infectious myalgic encephalomyelitis/chronic fatigue syndrome: A narrative review, Ther. Adv. Infect. Dis
Qin, Cao, Wen, Qingsong, Chaoyong, An antioxidant enzyme therapeutic for COVID-19, Adv. Mater
Rajinikanth, Venkatachalam, Manavalan, Investigations on the potential of serratiopeptidase-a proteolytic enzyme, on acetic acid induced ulcerative colitis in mice, Int. J. Pharm. Pharmaceut. Sci
Rao, Bested, Beaulne, Katzman, Iorio et al., A randomized, double-blind, placebocontrolled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome, Gut. Pathogens, doi:10.1186/1757-4749-1-6
Raveendran, Jayadevan, Sashidharan, Long COVID: An overview, diabetes & metabolic syndrome, Clin. Res. Rev
Rosa, De, Tewary, Varadhachary, Oppenheim, Lactoferrin acts as an alarmin to promote the recruitment and activation of antigen-presenting cells and antigen-specific immune responses, J. Immunol, doi:10.4049/jimmunol.180.10.6868
Rose, Herder, Löffler, Meierhoff, Schloot et al., Dose-dependent induction of IL-6 by plant-derived proteases in vitro, Clin. Exp. Immunol, doi:10.1111/j.1365-2249.2005.02970.x
Rudroff, Fietsam, Deters, Bryant, Kamholz, Post-COVID-19 fatigue: Potential contributing factors, Brain Sci, doi:10.3390/brainsci10121012
Sagar, Rathinavel, Lutz, Struble, Khurana et al., Bromelain inhibits SARS-CoV-2 infection in VeroE6 cells, bioRxiv, doi:10.1101/2020.09.16.297366
Shah, Parate, Vispute, Potential of the Combination of a systemic enzyme complex and probiotics administration to combat COVID-19: A randomized open label prospective analysis, Adv. Clin. Toxicol
Shi, Shi, Feng, Ye, Zhu et al., High expression of recombinant human catalase and its immunomodulatory effects on H1N1 influenza virus infection, Process. Biochem, doi:10.1016/j.procbio.2013.01.002
Singh, Naidu, Gupta, Kulkarni, Effect of natural and synthetic antioxidants in a mouse model of chronic fatigue syndrome, J. Med. Food, doi:10.1089/109662002763003366
Sullivan, Nord, Evengard, Effect of supplement with lactic-acid producing bacteria on fatigue and physical activity in patients with chronic fatigue syndrome, Nutr. J, doi:10.1186/1475-2891-8-4
Tansey, Louie, Loeb, Gold, Muller et al., One-year outcomes and health care utilization in survivors of severe acute respiratory syndrome, Arch. Intern. Med, doi:10.1001/archinte.167.12.1312
Toogood, Clauw, Phadke, Hoffman, Myalgic encephalomyelitis/ chronic fatigue syndrome (ME/CFS): Where will the drugs come from?, Pharmacol. Res, doi:10.1016/j.phrs.2021.105465
Townsend, Dyer, Jones, Dunne, Mooney et al., Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection, PLoS ONE
Venturini, Bacchi, Capelli, Lorusso, Ricevuti et al., Modification of immunological parameters, oxidative stress markers, mood symptoms, and well-being status in CFS patients after probiotic intake: Observations from a pilot study, Oxid. Med. Cel. Longev, doi:10.1155/2019/1684198
Vollbracht, Kraft, Feasibility of vitamin C in the treatment of post viral fatigue with focus on long COVID, based on a systematic review of IV vitamin C on fatigue, Nutrients, doi:10.3390/nu13041154
Wang, Wu, Wang, Antioxidant properties of probiotic bacteria, Nutrients, doi:10.3390/nu9050521
Williamson, Pizano, Gasta, Probiotics and disease: A comprehensive summary-Part 3, Cardiometabolic disease and fatigue syndromes, Integr. Med
Wilson, Concern coronavirus may trigger post-viral fatigue syndromes, New Sci, doi:10.1016/S0262-4079(20)30746-6
Xu, Wu, Jiang, Xu, Ying et al., Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: Retrospective case series, BMJ, doi:10.1136/bmj.m606
Yelin, Wirtheim, Vetter, Kalil, Bruchfeld et al., Long-term consequences of COVID-19: Research needs, Lancet. infect. Dis, doi:10.1016/S1473-3099(20)30701-5
DOI record:
{
"DOI": "10.3390/medicines8090047",
"ISSN": [
"2305-6320"
],
"URL": "http://dx.doi.org/10.3390/medicines8090047",
"abstract": "<jats:p>Muscle fatigue and cognitive disturbances persist in patients after recovery from acute COVID-19 disease. However, there are no specific treatments for post-COVID fatigue. Objective: To evaluate the efficacy and safety of the health supplements ImmunoSEB (systemic enzyme complex) and ProbioSEB CSC3 (probiotic complex) in patients suffering from COVID-19 induced fatigue. A randomized, multicentric, double blind, placebo-controlled trial was conducted in 200 patients with a complaint of post-COVID fatigue. The test arm (n = 100) received the oral supplements for 14 days and the control arm (n = 100) received a placebo. Treatment efficacy was compared using the Chalder Fatigue scale (CFQ-11), at various time points from days 1 to 14. The supplemental treatment resulted in resolution of fatigue in a greater percentage of subjects in the test vs. the control arm (91% vs. 15%) on day 14. Subjects in the test arm showed a significantly greater reduction in total as well as physical and mental fatigue scores at all time points vs. the control arm. The supplements were well tolerated with no adverse events reported. This study demonstrates that a 14 days supplementation of ImmunoSEB + ProbioSEB CSC3 resolves post-COVID-19 fatigue and can improve patients’ functional status and quality of life.</jats:p>",
"alternative-id": [
"medicines8090047"
],
"author": [
{
"affiliation": [],
"family": "Rathi",
"given": "Abhijit",
"sequence": "first"
},
{
"affiliation": [],
"family": "Jadhav",
"given": "Swati B.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9286-3801",
"affiliation": [],
"authenticated-orcid": false,
"family": "Shah",
"given": "Neha",
"sequence": "additional"
}
],
"container-title": "Medicines",
"container-title-short": "Medicines",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
8,
31
]
],
"date-time": "2021-08-31T02:49:51Z",
"timestamp": 1630378191000
},
"deposited": {
"date-parts": [
[
2021,
8,
31
]
],
"date-time": "2021-08-31T03:30:00Z",
"timestamp": 1630380600000
},
"indexed": {
"date-parts": [
[
2022,
5,
9
]
],
"date-time": "2022-05-09T17:30:16Z",
"timestamp": 1652117416611
},
"is-referenced-by-count": 5,
"issue": "9",
"issued": {
"date-parts": [
[
2021,
8,
30
]
]
},
"journal-issue": {
"issue": "9",
"published-online": {
"date-parts": [
[
2021,
9
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
8,
30
]
],
"date-time": "2021-08-30T00:00:00Z",
"timestamp": 1630281600000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2305-6320/8/9/47/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "47",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2021,
8,
30
]
]
},
"published-online": {
"date-parts": [
[
2021,
8,
30
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"article-title": "Long COVID: An overview, diabetes & metabolic syndrome",
"author": "Raveendran",
"first-page": "869",
"journal-title": "Clin. Res. Rev.",
"key": "ref1",
"volume": "15",
"year": "2021"
},
{
"DOI": "10.3390/brainsci10121012",
"doi-asserted-by": "publisher",
"key": "ref2"
},
{
"DOI": "10.1136/bmj.m606",
"doi-asserted-by": "publisher",
"key": "ref3"
},
{
"DOI": "10.1371/journal.pone.0240784",
"doi-asserted-by": "publisher",
"key": "ref4"
},
{
"key": "ref5"
},
{
"DOI": "10.1016/j.jchf.2020.06.001",
"doi-asserted-by": "publisher",
"key": "ref6"
},
{
"DOI": "10.1101/2021.02.06.21249256",
"doi-asserted-by": "publisher",
"key": "ref7"
},
{
"article-title": "COVID-19 and post-infectious myalgic encephalomyelitis/chronic fatigue syndrome: A narrative review",
"author": "Poenaru",
"first-page": "20499361211009385",
"journal-title": "Ther. Adv. Infect. Dis.",
"key": "ref8",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1016/j.mehy.2020.110055",
"doi-asserted-by": "publisher",
"key": "ref9"
},
{
"DOI": "10.1016/j.phrs.2021.105465",
"doi-asserted-by": "publisher",
"key": "ref10"
},
{
"article-title": "Post COVID-19 persistent fatigue: A proposal for rehabilitative interventions in the spa setting",
"author": "Maccarone",
"first-page": "1",
"journal-title": "Intern. J. Biometeorol.",
"key": "ref11",
"year": "2021"
},
{
"DOI": "10.1186/1757-4749-1-6",
"doi-asserted-by": "publisher",
"key": "ref12"
},
{
"DOI": "10.1155/2019/1684198",
"doi-asserted-by": "publisher",
"key": "ref13"
},
{
"DOI": "10.1089/109662002763003366",
"doi-asserted-by": "publisher",
"key": "ref14"
},
{
"DOI": "10.1016/j.imbio.2008.04.003",
"doi-asserted-by": "publisher",
"key": "ref15"
},
{
"DOI": "10.1016/j.jaim.2017.08.008",
"doi-asserted-by": "publisher",
"key": "ref16"
},
{
"DOI": "10.1111/j.1365-2249.2005.02970.x",
"doi-asserted-by": "publisher",
"key": "ref17"
},
{
"DOI": "10.4049/jimmunol.180.10.6868",
"doi-asserted-by": "publisher",
"key": "ref18"
},
{
"DOI": "10.1023/A:1007936629501",
"doi-asserted-by": "publisher",
"key": "ref19"
},
{
"DOI": "10.1016/j.procbio.2013.01.002",
"doi-asserted-by": "publisher",
"key": "ref20"
},
{
"DOI": "10.1152/ajpendo.00349.2005",
"doi-asserted-by": "publisher",
"key": "ref21"
},
{
"DOI": "10.1016/j.btre.2020.e00544",
"doi-asserted-by": "publisher",
"key": "ref22"
},
{
"article-title": "Management of post COVID-19 fatigue using systemic enzymes and probiotics—Case series",
"author": "Parate",
"first-page": "281",
"journal-title": "Med. J. Clin. Trials Case Stud.",
"key": "ref23",
"volume": "5",
"year": "2021"
},
{
"DOI": "10.1136/jnnp.54.2.153",
"doi-asserted-by": "publisher",
"key": "ref24"
},
{
"DOI": "10.1016/0022-3999(93)90081-P",
"doi-asserted-by": "publisher",
"key": "ref25"
},
{
"DOI": "10.1136/bmj.m2815",
"doi-asserted-by": "publisher",
"key": "ref26"
},
{
"DOI": "10.1016/S1473-3099(20)30701-5",
"doi-asserted-by": "publisher",
"key": "ref27"
},
{
"DOI": "10.1016/S0262-4079(20)30746-6",
"doi-asserted-by": "publisher",
"key": "ref28"
},
{
"DOI": "10.1038/d41586-020-02598-6",
"doi-asserted-by": "publisher",
"key": "ref29"
},
{
"DOI": "10.1001/archinte.167.12.1312",
"doi-asserted-by": "publisher",
"key": "ref30"
},
{
"DOI": "10.7861/clinmed.2020-0204",
"doi-asserted-by": "publisher",
"key": "ref31"
},
{
"DOI": "10.30773/pi.2018.10.22.3",
"doi-asserted-by": "publisher",
"key": "ref32"
},
{
"DOI": "10.1093/occmed/kql169",
"doi-asserted-by": "publisher",
"key": "ref33"
},
{
"DOI": "10.1016/S0022-3999(98)00022-1",
"doi-asserted-by": "publisher",
"key": "ref34"
},
{
"DOI": "10.1016/S0022-3999(97)00291-2",
"doi-asserted-by": "publisher",
"key": "ref35"
},
{
"DOI": "10.1093/occmed/kqu168",
"doi-asserted-by": "publisher",
"key": "ref36"
},
{
"DOI": "10.3390/nu13041154",
"doi-asserted-by": "publisher",
"key": "ref37"
},
{
"DOI": "10.1007/s11926-017-0628-x",
"doi-asserted-by": "publisher",
"key": "ref38"
},
{
"DOI": "10.1186/1475-2891-8-4",
"doi-asserted-by": "publisher",
"key": "ref39"
},
{
"article-title": "The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems",
"author": "Carabotti",
"first-page": "203",
"journal-title": "Ann. Gastroenterol.",
"key": "ref40",
"volume": "28",
"year": "2015"
},
{
"article-title": "Investigations on the potential of serratiopeptidase– a proteolytic enzyme, on acetic acid induced ulcerative colitis in mice",
"author": "Rajinikanth",
"first-page": "525",
"journal-title": "Int. J. Pharm. Pharmaceut. Sci.",
"key": "ref41",
"volume": "6",
"year": "2014"
},
{
"DOI": "10.1159/000056191",
"doi-asserted-by": "publisher",
"key": "ref42"
},
{
"DOI": "10.4049/jimmunol.181.12.8382",
"doi-asserted-by": "publisher",
"key": "ref43"
},
{
"DOI": "10.1021/acs.bioconjchem.7b00719",
"doi-asserted-by": "publisher",
"key": "ref44"
},
{
"DOI": "10.1186/s12917-019-2067-6",
"doi-asserted-by": "publisher",
"key": "ref45"
},
{
"DOI": "10.1101/2020.09.16.297366",
"doi-asserted-by": "publisher",
"key": "ref46"
},
{
"DOI": "10.20944/preprints202012.0543.v1",
"doi-asserted-by": "publisher",
"key": "ref47"
},
{
"DOI": "10.1002/adma.202004901",
"doi-asserted-by": "publisher",
"key": "ref48"
},
{
"DOI": "10.1155/2018/8063647",
"doi-asserted-by": "publisher",
"key": "ref49"
},
{
"DOI": "10.1186/s12974-019-1611-4",
"doi-asserted-by": "publisher",
"key": "ref50"
},
{
"DOI": "10.3390/nu9050521",
"doi-asserted-by": "publisher",
"key": "ref51"
},
{
"DOI": "10.1038/s41598-019-39602-7",
"doi-asserted-by": "publisher",
"key": "ref52"
},
{
"DOI": "10.1007/s12275-018-8032-4",
"doi-asserted-by": "publisher",
"key": "ref53"
},
{
"DOI": "10.3389/fpsyt.2019.00164",
"doi-asserted-by": "publisher",
"key": "ref54"
},
{
"article-title": "Probiotics and disease: A comprehensive summary—Part 3, Cardiometabolic disease and fatigue syndromes",
"author": "Williamson",
"first-page": "30",
"journal-title": "Integr. Med.",
"key": "ref55",
"volume": "16",
"year": "2017"
},
{
"DOI": "10.1016/j.jad.2017.11.063",
"doi-asserted-by": "publisher",
"key": "ref56"
},
{
"article-title": "Potential of the Combination of a systemic enzyme complex and probiotics administration to combat COVID-19: A randomized open label prospective analysis",
"author": "Shah",
"first-page": "205",
"journal-title": "Adv. Clin. Toxicol.",
"key": "ref57",
"volume": "6",
"year": "2021"
}
],
"reference-count": 57,
"references-count": 57,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2305-6320/8/9/47"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "A Randomized Controlled Trial of the Efficacy of Systemic Enzymes and Probiotics in the Resolution of Post-COVID Fatigue",
"type": "journal-article",
"volume": "8"
}
