Sodium Bicarbonate Nebulized Therapy in Patients with Confirmed COVID-19
et al., Advanced Pharmaceutical Bulletin, doi:10.34172/apb.2021.047, Oct 2020
32nd treatment shown to reduce risk in
November 2021, now with p = 0.0000000039 from 14 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
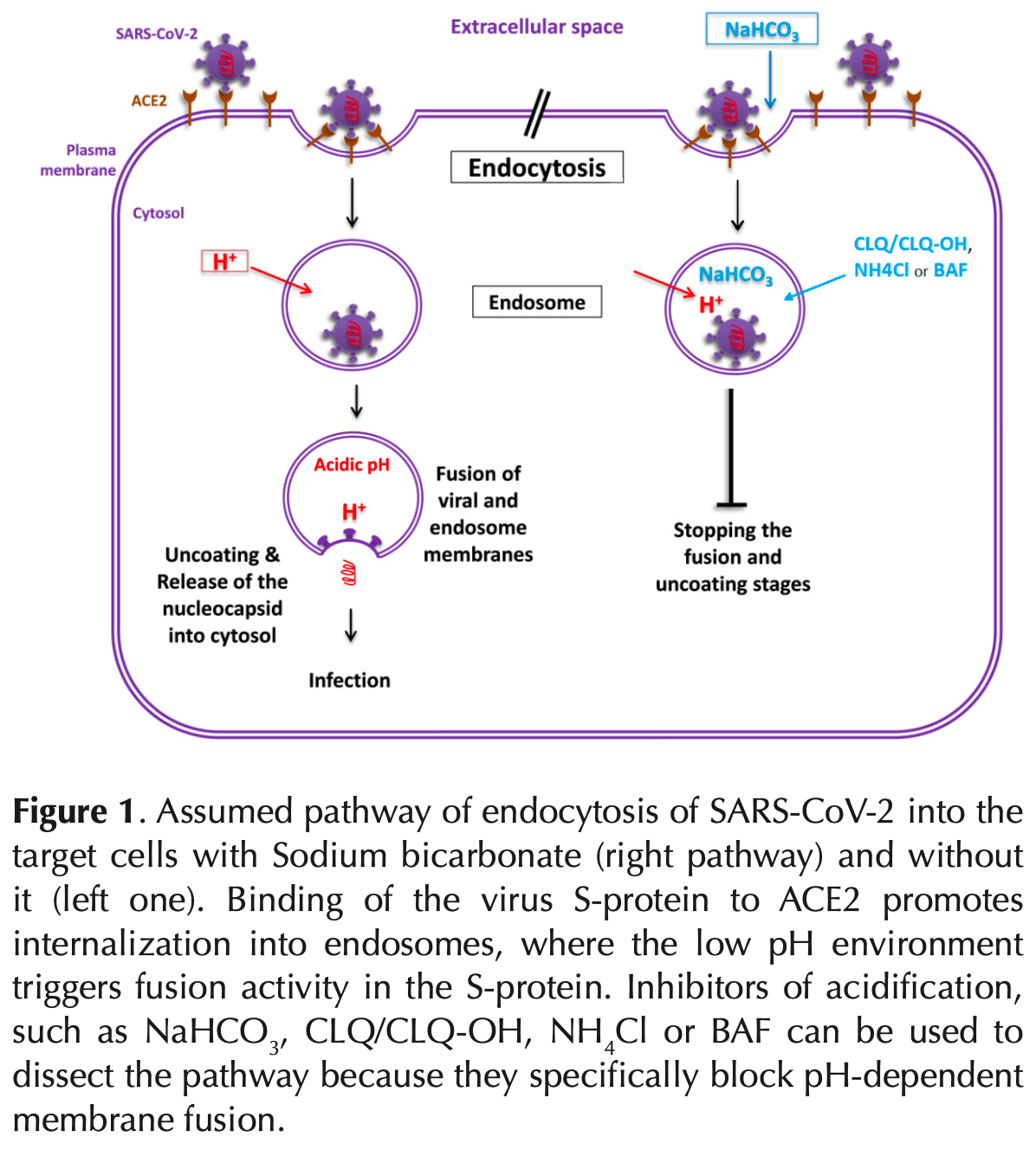
Proposal for nebulized sodium bicarbonate therapy for prevention of SARS-CoV-2 infection by raising endosomal pH and inhibiting viral entry into cells. Author proposes that inhalation of nebulized sodium bicarbonate solution (<5%) several times per day by COVID-19 patients may stop viral fusion and uncoating stages. Author notes that prospective controlled trials are needed.
1.
Donzelli, A., Neglected Effective Early Therapies against COVID-19: Focus on Functional Foods and Related Active Substances. A Review, MDPI AG, doi:10.20944/preprints202312.1178.v1.
Rashedi et al., 14 Oct 2020, peer-reviewed, 3 authors.
Sodium Bicarbonate Nebulized Therapy in Patients with Confirmed COVID-19
Advanced Pharmaceutical Bulletin, doi:10.34172/apb.2021.047
replication phase will also remain barren and eventually the respiratory infection will be controlled. Prospective controlled trials are needed to evaluate this method efficacy.
Ethical Issues Not applicable.
Conflict of Interest The authors declare that there is no conflict of interests.
References
Belouzard, Millet, Licitra, Whittaker, None
Hofmann, Pöhlmann, Cellular entry of the SARS coronavirus, Trends Microbiol, doi:10.1016/j.tim.2004.08.008
DOI record:
{
"DOI": "10.34172/apb.2021.047",
"ISSN": [
"2228-5881",
"2251-7308"
],
"URL": "http://dx.doi.org/10.34172/apb.2021.047",
"assertion": [
{
"label": "Journal Owner",
"name": "journal_owner",
"value": "Tabriz University of Medical Sciences"
},
{
"label": "Journal Publisher",
"name": "journal_publisher",
"value": "Tabriz University of Medical Sciences"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "2020-05-09"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "2020-10-14"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 3,
"value": "2020-10-14"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-9627-1491",
"affiliation": [
{
"name": "Department of Laboratory Sciences, School of Paramedicine, Tabriz University of Medical Sciences, Tabriz, Iran."
}
],
"authenticated-orcid": true,
"family": "Rashedi",
"given": "Jalil",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-8856-3750",
"affiliation": [
{
"name": "Department of Laboratory Sciences, School of Paramedicine, Tabriz University of Medical Sciences, Tabriz, Iran."
}
],
"authenticated-orcid": true,
"family": "Mahdavi Poor",
"given": "Behroz",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3386-4342",
"affiliation": [
{
"name": "Biotechnology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran."
}
],
"authenticated-orcid": true,
"family": "Asgharzadeh",
"given": "Mohammad",
"sequence": "additional"
}
],
"container-title": "Advanced Pharmaceutical Bulletin",
"container-title-short": "Adv Pharm Bull",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"apb.tbzmed.ac.ir"
]
},
"created": {
"date-parts": [
[
2021,
7,
5
]
],
"date-time": "2021-07-05T15:12:03Z",
"timestamp": 1625497923000
},
"deposited": {
"date-parts": [
[
2021,
7,
5
]
],
"date-time": "2021-07-05T15:12:03Z",
"timestamp": 1625497923000
},
"indexed": {
"date-parts": [
[
2024,
2,
7
]
],
"date-time": "2024-02-07T21:01:14Z",
"timestamp": 1707339674306
},
"is-referenced-by-count": 2,
"issue": "3",
"issued": {
"date-parts": [
[
2020,
10,
14
]
]
},
"journal-issue": {
"issue": "3",
"published-online": {
"date-parts": [
[
2021,
5
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://apb.tbzmed.ac.ir/PDF/apb-11-397.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://apb.tbzmed.ac.ir/PDF/apb-11-397.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "20123",
"original-title": [],
"page": "397-398",
"prefix": "10.34172",
"published": {
"date-parts": [
[
2020,
10,
14
]
]
},
"published-online": {
"date-parts": [
[
2020,
10,
14
]
]
},
"published-print": {
"date-parts": [
[
2020,
10,
14
]
]
},
"publisher": "Maad Rayan Publishing Company",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://apb.tbzmed.ac.ir/Article/apb-28931"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Sodium Bicarbonate Nebulized Therapy in Patients with Confirmed COVID-19",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.34172/crossmark_policy",
"volume": "11"
}
