Development of a trispecific fusion protein based on angiotensin-converting enzyme 2, glycoprotein 130, and tumor necrosis factor receptor 2 as a promising therapeutic for COVID-19
et al., Molecular Biomedicine, doi:10.1186/s43556-025-00320-4, Oct 2025
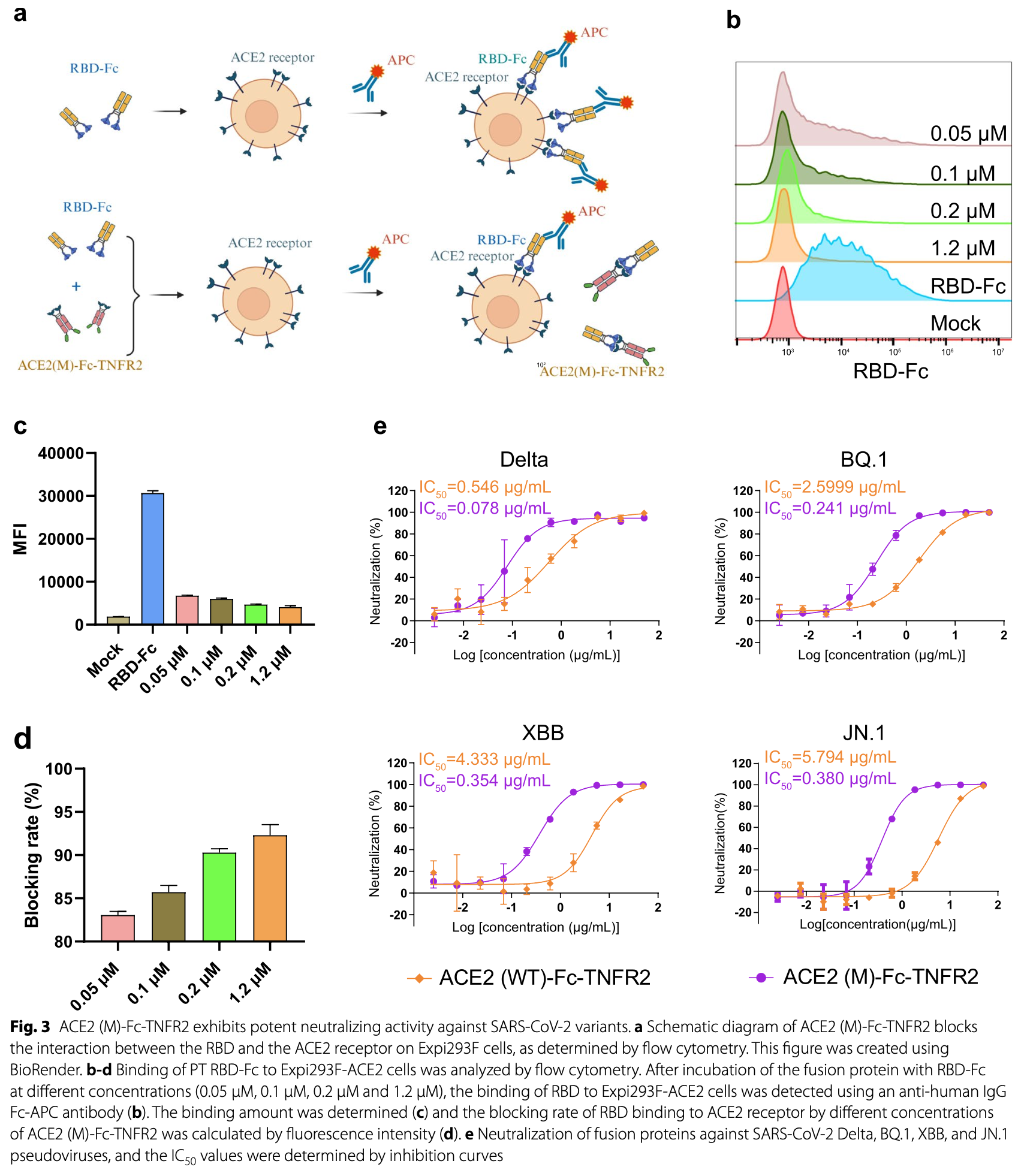
In vitro and mouse study showing that trispecific fusion proteins targeting ACE2, GP130, and TNFR2 provide dual anti-viral and anti-inflammatory benefits for COVID-19 treatment.
Qiao et al., 15 Oct 2025, peer-reviewed, 20 authors.
Contact: chunbo_dong@saari.org.cn, wanghaidong@sxau.edu.cn, zhida_liu@saari.org.cn, 101013216@seu.edu.cn.
Development of a trispecific fusion protein based on angiotensin-converting enzyme 2, glycoprotein 130, and tumor necrosis factor receptor 2 as a promising therapeutic for COVID-19
Molecular Biomedicine, doi:10.1186/s43556-025-00320-4
Despite a substantial reduction in the incidence of coronavirus disease 2019 (COVID-19) infections, severe cases continue to pose a significant clinical burden, particularly among elderly individuals and patients with underlying medical conditions, due to high viral loads and cytokine storm syndrome. Elevated levels of interleukin-6 (IL-6) and tumor necrosis factor (TNF), signaling through their respective receptors, glycoprotein 130 (GP130)/interleukin-6 receptor (IL-6R) and tumor necrosis factor receptor 2 (TNFR2), are independent predictors of disease severity and mortality. To address this challenge, a series of bifunctional and trifunctional decoy receptor fusion proteins were developed by fusing the extracellular domains of TNFR2 and/or GP130 to an engineered angiotensin-converting enzyme 2 (ACE2) protein, the entry receptor for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Five mutations (T27F, K31Y, L79W, R273Q, and N330Y) were introduced into the ACE2 domain to enhance its binding affinity and neutralizing activity against a broad range of SARS-CoV-2 variants, including the currently circulating JN.1 variant. The TNFR2 and GP130 domain confer strong binding to TNF and IL-6R-IL-6 complex, respectively, thereby effectively blocking pro-inflammatory signaling pathways. In a mouse model of acute lung inflammation induced by R848, treatment with the bifunctional and trifunctional fusion proteins markedly attenuated pulmonary pathology by dampening IL-6-and TNF-mediated inflammation. These findings demonstrate a promising therapeutic strategy for severe COVID-19 and offer a framework for designing multifunctional biologics against emerging viral infections.
Supplementary Information The online version contains supplementary material available at https:// doi. org/ 10. 1186/ s43556-025-00320-4 . Supplementary Material 1.
Authors' contributions
Declarations Ethics approval and consent to participate Animal studies were conducted following approval from the Research Ethics Committee of the Institute of Microbiology, Chinese Academy of Sciences (APIMCAS2022124) and Institutional Animal Care and Use Committee of Shanxi Agricultural University (SXAU-EAW-2023M.DF.001017216). The studies were conducted in accordance with the local legislation and institutional requirements.
Consent for publication Not applicable.
Competing interests Competing interests Z.L., P.H., C.D., Y.Q., Y.H., H.H. and L.Z. have filed a patent for protecting a bifunctional therapeutic protein ACE2 (M)-Fc-GP130-TNFR2 for the treatment of patients with SARS-CoV-2 infection. Author Hong Hu is an employee in Ankerui (Shanxi) Biological Cell Co, but has no potential relevant financial or non-financial interests to disclose. The other authors have no conflicts of interest to declare.
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Abbasi, What to Know About EG.5, the Latest SARS-CoV-2 "Variant of Interest, JAMA, doi:10.1001/jama.2023.16498
Berg, Ettich, Weitz, Krusche, Floss et al., Exclusive inhibition of IL-6 trans-signaling by soluble gp130(FlyR)Fc, Cytokine X, doi:10.1016/j.cytox.2021.100058
Bernal, Da Silva, Musungaie, Kovalchuk, Gonzalez et al., Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients, N Engl J Med, doi:10.1056/NEJMoa2116044
Chan, Dorosky, Sharma, Abbasi, Dye et al., Engineering human ACE2 to optimize binding to the spike protein of SARS coronavirus 2, Science, doi:10.1126/science.abc0870
Chen, Sun, Ullah, Beaudoin-Bussières, Anand et al., Engineered ACE2-Fc counters murine lethal SARS-CoV-2 infection through direct neutralization and Fc-effector activities, Sci Adv, doi:10.1126/sciadv.abn4188
Chen, Zhang, Li, Liu, Dong et al., Bat-infecting merbecovirus HKU5-CoV lineage 2 can use human ACE2 as a cell entry receptor, Cell, doi:10.1016/j.cell.2025.01.042
Dejnirattisai, Huo, Zhou, Zahradník, Supasa et al., SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses, Cell, doi:10.1016/j.cell.2021.12.046
Elkind, Harrington, Benjamin, The Role of the American Heart Association in the Global COVID-19 Pandemic, Circulation, doi:10.1161/circulationaha.120.046749
Feldmann, Maini, Anti-TNF alpha therapy of rheumatoid arthritis: what have we learned?, Annu Rev Immunol, doi:10.1146/annurev.immunol.19.1.163
Ferrari, Mekkaoui, Ilca, Akbar, Bughda et al., Characterization of a novel ACE2-based therapeutic with enhanced rather than reduced activity against SARS-CoV-2 variants, J Virol, doi:10.1128/jvi.00685-21
Fischer, Goldschmitt, Peschel, Brakenhoff, Kallen et al., A bioactive designer cytokine for human hematopoietic progenitor cell expansion, Nat Biotechnol, doi:10.1038/nbt0297-142
Hadjadj, Yatim, Barnabei, Corneau, Boussier et al., Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients, Science, doi:10.1126/science.abc6027
Han, Ma, Li, Liu, Zhao et al., Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors, Emerg Microbes Infect, doi:10.1080/22221751.2020.1770129
Hibi, Murakami, Saito, Hirano, Taga et al., Molecular cloning and expression of an IL-6 signal transducer, gp130, Cell, doi:10.1016/0092-8674(90)90411-7
Higuchi, Suzuki, Arimori, Ikemura, Mihara et al., Engineered ACE2 receptor therapy overcomes mutational escape of SARS-CoV-2, Nat Commun, doi:10.1038/s41467-021-24013-y
Hofmann, Pyrc, Van Der Hoek, Geier, Berkhout et al., Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry, Proc Natl Acad Sci U S A, doi:10.1073/pnas.0409465102
Hu, Huang, Yin, The cytokine storm and COVID-19, J Med Virol, doi:10.1002/jmv.26232
Huang, Wang, Li, Ren, Zhao et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet, doi:10.1016/s0140-6736(20)30183-5
Iwanaga, Cooper, Rong, Maness, Beddingfield et al., ACE2-IgG1 fusions with improved in vitro and in vivo activity against SARS-CoV-2, iScience, doi:10.1016/j.isci.2021.103670
Jostock, Müllberg, Ozbek, Atreya, Blinn et al., Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses, Eur J Biochem, doi:10.1046/j.1432-1327.2001.01867.x
Kalil, Patterson, Mehta, Tomashek, Wolfe et al., Baricitinib plus remdesivir for hospitalized adults with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2031994
Liu, Conceptualization, Resources, Supervision, Project administration, Funding acquisition, Writing-review & editing ; Pengcheng Han: Conceptualization, Resources, Supervision, Project administration, Funding acquisition, Writing-review & editing
Meehan, Herder, Allan, Huang, Kerr et al., Phenotyping the virulence of SARS-CoV-2 variants in hamsters by digital pathology and machine learning, PLoS Pathog, doi:10.1371/journal.ppat.1011589
Mehta, Mcauley, Brown, Sanchez, Tattersall et al., COVID-19: consider cytokine storm syndromes and immunosuppression, Lancet, doi:10.1016/s0140-6736(20)30628-0
Moore, June, Cytokine release syndrome in severe COVID-19, Science, doi:10.1126/science.abb8925
Rose-John, Jenkins, Garbers, Moll, Scheller, Targeting IL-6 trans-signalling: past, present and future prospects, Nat Rev Immunol, doi:10.1038/s41577-023-00856-y
Ruwanpura, Mcleod, Dousha, Seow, Alhayyani et al., Therapeutic targeting of the IL-6 trans-signaling/mechanistic target of rapamycin complex 1 axis in pulmonary emphysema, Am J Respir Crit Care Med, doi:10.1164/rccm.201512-2368OC
Sardu, Gargiulo, Esposito, Paolisso, Marfella, Impact of diabetes mellitus on clinical outcomes in patients affected by Covid-19, Cardiovasc Diabetol, doi:10.1186/s12933-020-01047-y
Sauerborn, Brinks, Jiskoot, Schellekens, Immunological mechanism underlying the immune response to recombinant human protein therapeutics, Trends Pharmacol Sci, doi:10.1016/j.tips.2009.11.001
Schreiber, Aden, Bernardes, Conrad, Tran et al., Therapeutic Interleukin-6 Trans-signaling Inhibition by Olamkicept (sgp130Fc) in Patients With Active Inflammatory Bowel Disease, Gastroenterology, doi:10.1053/j.gastro.2021.02.062
Spinner, Gottlieb, Criner, López, Cattelan et al., Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial, JAMA, doi:10.1001/jama.2020.16349
Taga, Hibi, Hirata, Yamasaki, Yasukawa et al., Interleukin-6 triggers the association of its receptor with a possible signal transducer, gp130, Cell, doi:10.1016/0092-8674(89)90438-8
Tan, Guo, Fang, Wang, Li et al., Characterization and comparison of commercially available TNF receptor 2-Fc fusion protein products, MAbs, doi:10.4161/mabs.22276
Tan, Zhu, Chia, Young, Yeoh et al., Distinctive serotypes of SARS-related coronaviruses defined by convalescent sera from unvaccinated individuals, Hlife, doi:10.1016/j.hlife.2023.07.002
Teachey, Lacey, Shaw, Melenhorst, Maude et al., Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia, Cancer Discov, doi:10.1158/2159-8290.Cd-16-0040
Temmam, Vongphayloth, Baquero, Munier, Bonomi et al., Bat coronaviruses related to SARS-CoV-2 and infectious for human cells, Nature, doi:10.1038/s41586-022-04532-4
Valle, Kim-Schulze, Huang, Beckmann, Nirenberg et al., An inflammatory cytokine signature predicts COVID-19 severity and survival, Nat Med, doi:10.1038/s41591-020-1051-9
Van Dam, Müllberg, Schooltink, Stoyan, Brakenhoff et al., Structure-function analysis of interleukin-6 utilizing human/murine chimeric molecules. Involvement of two separate domains in receptor binding, J Biol Chem
Wang, Conceptualization, Resources, Supervision, Writing-review & editing
Wang, Iketani, Li, Liu, Guo et al., Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants, Cell, doi:10.1016/j.cell.2022.12.018
Wang, Jiang, Jiang, Li, Lin, Neutralization of SARS-CoV-2 BQ.1.1, CH.1.1, and XBB.1.5 by breakthrough infection sera from previous and recent waves in China, Cell Discov, doi:10.1038/s41421-023-00569-5
Wang, Zhang, Wu, Niu, Song et al., Structural and functional basis of SARS-CoV-2 entry by using human ACE2, Cell, doi:10.1016/j.cell.2020.03.045
Wu, Data curation, Resources, Investigation
Zhang, Lv, Jiang, Liu, Yan et al., Advances in developing ACE2 derivatives against SARS-CoV-2, Lancet Microbe, doi:10.1016/s2666-5247(23)00011-3
Zhang, Xiao, Cai, Lavine, Peng et al., Membrane fusion and immune evasion by the spike protein of SARS-CoV-2 Delta variant, Science, doi:10.1126/science.abl9463
Zhang, Zeng, Zhang, Sun, Potent prophylactic and therapeutic efficacy of recombinant human ACE2-Fc against SARS-CoV-2 infection in vivo, Cell Discov, doi:10.1038/s41421-021-00302-0
Zheng, Wu, Ma, Han, Huang et al., A binding-enhanced but enzymatic activity-eliminated human ACE2 efficiently neutralizes SARS-CoV-2 variants, Signal Transduct Target Ther, doi:10.1038/s41392-021-00821-y
Zhou, Yu, Du, Fan, Liu et al., Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, Lancet, doi:10.1016/s0140-6736(20)30566-3
Zoufaly, Poglitsch, Aberle, Hoepler, Seitz et al., Human recombinant soluble ACE2 in severe COVID-19, Lancet Respir Med, doi:10.1016/s2213-2600(20)30418-5
DOI record:
{
"DOI": "10.1186/s43556-025-00320-4",
"ISSN": [
"2662-8651"
],
"URL": "http://dx.doi.org/10.1186/s43556-025-00320-4",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:p>Despite a substantial reduction in the incidence of coronavirus disease 2019 (COVID-19) infections, severe cases continue to pose a significant clinical burden, particularly among elderly individuals and patients with underlying medical conditions, due to high viral loads and cytokine storm syndrome. Elevated levels of interleukin-6 (IL-6) and tumor necrosis factor (TNF), signaling through their respective receptors, glycoprotein 130 (GP130)/interleukin-6 receptor (IL-6R) and tumor necrosis factor receptor 2 (TNFR2), are independent predictors of disease severity and mortality. To address this challenge, a series of bifunctional and trifunctional decoy receptor fusion proteins were developed by fusing the extracellular domains of TNFR2 and/or GP130 to an engineered angiotensin-converting enzyme 2 (ACE2) protein, the entry receptor for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Five mutations (T27F, K31Y, L79W, R273Q, and N330Y) were introduced into the ACE2 domain to enhance its binding affinity and neutralizing activity against a broad range of SARS-CoV-2 variants, including the currently circulating JN.1 variant. The TNFR2 and GP130 domain confer strong binding to TNF and IL-6R-IL-6 complex, respectively, thereby effectively blocking pro-inflammatory signaling pathways. In a mouse model of acute lung inflammation induced by R848, treatment with the bifunctional and trifunctional fusion proteins markedly attenuated pulmonary pathology by dampening IL-6– and TNF–mediated inflammation. These findings demonstrate a promising therapeutic strategy for severe COVID-19 and offer a framework for designing multifunctional biologics against emerging viral infections.</jats:p>",
"alternative-id": [
"320"
],
"article-number": "74",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "20 April 2025"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Revised",
"name": "revised",
"order": 2,
"value": "5 September 2025"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 3,
"value": "9 September 2025"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 4,
"value": "15 October 2025"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "Animal studies were conducted following approval from the Research Ethics Committee of the Institute of Microbiology, Chinese Academy of Sciences (APIMCAS2022124) and Institutional Animal Care and Use Committee of Shanxi Agricultural University (SXAU-EAW-2023M.DF.001017216). The studies were conducted in accordance with the local legislation and institutional requirements."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Not applicable."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "Competing interests Z.L., P.H., C.D., Y.Q., Y.H., H.H. and L.Z. have filed a patent for protecting a bifunctional therapeutic protein ACE2 (M)-Fc-GP130-TNFR2 for the treatment of patients with SARS-CoV-2 infection. Author Hong Hu is an employee in Ankerui (Shanxi) Biological Cell Co, but has no potential relevant financial or non-financial interests to disclose. The other authors have no conflicts of interest to declare."
}
],
"author": [
{
"affiliation": [],
"family": "Qiao",
"given": "Yongfeng",
"sequence": "first"
},
{
"affiliation": [],
"family": "Han",
"given": "Yanjun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhao",
"given": "Lu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gao",
"given": "Wenjing",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hu",
"given": "Hong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Su",
"given": "Chao",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zheng",
"given": "Anqi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sun",
"given": "Junqing",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tian",
"given": "Mingxiong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wu",
"given": "Yarong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bai",
"given": "Lianmei",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lei",
"given": "Yuping",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wu",
"given": "Jiahao",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Weibing",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Han",
"given": "Pu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Xiaoyu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dong",
"given": "Chunbo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Haidong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liu",
"given": "Zhida",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Han",
"given": "Pengcheng",
"sequence": "additional"
}
],
"container-title": "Molecular Biomedicine",
"container-title-short": "Mol Biomed",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2025,
10,
14
]
],
"date-time": "2025-10-14T23:01:55Z",
"timestamp": 1760482915000
},
"deposited": {
"date-parts": [
[
2025,
10,
14
]
],
"date-time": "2025-10-14T23:01:56Z",
"timestamp": 1760482916000
},
"funder": [
{
"DOI": "10.13039/501100001809",
"award": [
"32222006"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100001809",
"id-type": "DOI"
}
],
"name": "National Natural Science Foundation of China"
}
],
"indexed": {
"date-parts": [
[
2025,
10,
14
]
],
"date-time": "2025-10-14T23:41:54Z",
"timestamp": 1760485314368,
"version": "build-2065373602"
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2025,
10,
15
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2025,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
10,
15
]
],
"date-time": "2025-10-15T00:00:00Z",
"timestamp": 1760486400000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
10,
15
]
],
"date-time": "2025-10-15T00:00:00Z",
"timestamp": 1760486400000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s43556-025-00320-4.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s43556-025-00320-4/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s43556-025-00320-4.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2025,
10,
15
]
]
},
"published-online": {
"date-parts": [
[
2025,
10,
15
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1186/s12933-020-01047-y",
"author": "C Sardu",
"doi-asserted-by": "publisher",
"first-page": "76",
"issue": "1",
"journal-title": "Cardiovasc Diabetol",
"key": "320_CR1",
"unstructured": "Sardu C, Gargiulo G, Esposito G, Paolisso G, Marfella R. Impact of diabetes mellitus on clinical outcomes in patients affected by Covid-19. Cardiovasc Diabetol. 2020;19(1):76. https://doi.org/10.1186/s12933-020-01047-y.",
"volume": "19",
"year": "2020"
},
{
"DOI": "10.1161/circulationaha.120.046749",
"author": "MSV Elkind",
"doi-asserted-by": "publisher",
"first-page": "e743",
"issue": "15",
"journal-title": "Circulation",
"key": "320_CR2",
"unstructured": "Elkind MSV, Harrington RA, Benjamin IJ. The Role of the American Heart Association in the Global COVID-19 Pandemic. Circulation. 2020;141(15):e743–5. https://doi.org/10.1161/circulationaha.120.046749.",
"volume": "141",
"year": "2020"
},
{
"DOI": "10.1016/s0140-6736(20)30566-3",
"author": "F Zhou",
"doi-asserted-by": "publisher",
"first-page": "1054",
"issue": "10229",
"journal-title": "Lancet",
"key": "320_CR3",
"unstructured": "Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–62. https://doi.org/10.1016/s0140-6736(20)30566-3.",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1158/2159-8290.Cd-16-0040",
"author": "DT Teachey",
"doi-asserted-by": "publisher",
"first-page": "664",
"issue": "6",
"journal-title": "Cancer Discov",
"key": "320_CR4",
"unstructured": "Teachey DT, Lacey SF, Shaw PA, Melenhorst JJ, Maude SL, Frey N, et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov. 2016;6(6):664–79. https://doi.org/10.1158/2159-8290.Cd-16-0040.",
"volume": "6",
"year": "2016"
},
{
"DOI": "10.1016/s0140-6736(20)30628-0",
"author": "P Mehta",
"doi-asserted-by": "publisher",
"first-page": "1033",
"issue": "10229",
"journal-title": "Lancet",
"key": "320_CR5",
"unstructured": "Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–4. https://doi.org/10.1016/s0140-6736(20)30628-0.",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/s0140-6736(20)30183-5",
"author": "C Huang",
"doi-asserted-by": "publisher",
"first-page": "497",
"issue": "10223",
"journal-title": "Lancet",
"key": "320_CR6",
"unstructured": "Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. https://doi.org/10.1016/s0140-6736(20)30183-5.",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.03.045",
"author": "Q Wang",
"doi-asserted-by": "publisher",
"first-page": "894",
"issue": "4",
"journal-title": "Cell",
"key": "320_CR7",
"unstructured": "Wang Q, Zhang Y, Wu L, Niu S, Song C, Zhang Z, et al. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020;181(4):894-904.e9. https://doi.org/10.1016/j.cell.2020.03.045.",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2022.12.018",
"author": "Q Wang",
"doi-asserted-by": "publisher",
"first-page": "279",
"issue": "2",
"journal-title": "Cell",
"key": "320_CR8",
"unstructured": "Wang Q, Iketani S, Li Z, Liu L, Guo Y, Huang Y, et al. Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell. 2023;186(2):279-86.e8. https://doi.org/10.1016/j.cell.2022.12.018.",
"volume": "186",
"year": "2023"
},
{
"DOI": "10.1126/science.abl9463",
"author": "J Zhang",
"doi-asserted-by": "publisher",
"first-page": "1353",
"issue": "6573",
"journal-title": "Science",
"key": "320_CR9",
"unstructured": "Zhang J, Xiao T, Cai Y, Lavine CL, Peng H, Zhu H, et al. Membrane fusion and immune evasion by the spike protein of SARS-CoV-2 Delta variant. Science. 2021;374(6573):1353–60. https://doi.org/10.1126/science.abl9463.",
"volume": "374",
"year": "2021"
},
{
"DOI": "10.1038/s41421-023-00569-5",
"author": "X Wang",
"doi-asserted-by": "publisher",
"issue": "1",
"journal-title": "Cell Discov",
"key": "320_CR10",
"unstructured": "Wang X, Jiang S, Jiang S, Li X, Ai J, Lin K, et al. Neutralization of SARS-CoV-2 BQ.1.1, CH.1.1, and XBB.1.5 by breakthrough infection sera from previous and recent waves in China. Cell Discov. 2023;9(1):64. https://doi.org/10.1038/s41421-023-00569-5.",
"volume": "9",
"year": "2023"
},
{
"DOI": "10.1016/j.hlife.2023.07.002",
"author": "CW Tan",
"doi-asserted-by": "publisher",
"first-page": "26",
"issue": "1",
"journal-title": "Hlife",
"key": "320_CR11",
"unstructured": "Tan CW, Zhu F, Chia WN, Young BE, Yeoh AYY, Althaus T, et al. Distinctive serotypes of SARS-related coronaviruses defined by convalescent sera from unvaccinated individuals. Hlife. 2023;1(1):26–34. https://doi.org/10.1016/j.hlife.2023.07.002.",
"volume": "1",
"year": "2023"
},
{
"DOI": "10.1016/s2666-5247(23)00011-3",
"author": "H Zhang",
"doi-asserted-by": "publisher",
"first-page": "e369",
"issue": "5",
"journal-title": "Lancet Microbe",
"key": "320_CR12",
"unstructured": "Zhang H, Lv P, Jiang J, Liu Y, Yan R, Shu S, et al. Advances in developing ACE2 derivatives against SARS-CoV-2. Lancet Microbe. 2023;4(5):e369–78. https://doi.org/10.1016/s2666-5247(23)00011-3.",
"volume": "4",
"year": "2023"
},
{
"DOI": "10.1038/s41421-021-00302-0",
"author": "Z Zhang",
"doi-asserted-by": "publisher",
"first-page": "65",
"issue": "1",
"journal-title": "Cell Discov",
"key": "320_CR13",
"unstructured": "Zhang Z, Zeng E, Zhang L, Wang W, Jin Y, Sun J, et al. Potent prophylactic and therapeutic efficacy of recombinant human ACE2-Fc against SARS-CoV-2 infection in vivo. Cell Discov. 2021;7(1):65. https://doi.org/10.1038/s41421-021-00302-0.",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.1126/sciadv.abn4188",
"author": "Y Chen",
"doi-asserted-by": "publisher",
"issue": "28",
"journal-title": "Sci Adv",
"key": "320_CR14",
"unstructured": "Chen Y, Sun L, Ullah I, Beaudoin-Bussières G, Anand SP, Hederman AP, et al. Engineered ACE2-Fc counters murine lethal SARS-CoV-2 infection through direct neutralization and Fc-effector activities. Sci Adv. 2022;8(28):eabn4188. https://doi.org/10.1126/sciadv.abn4188.",
"volume": "8",
"year": "2022"
},
{
"DOI": "10.1016/s2213-2600(20)30418-5",
"author": "A Zoufaly",
"doi-asserted-by": "publisher",
"first-page": "1154",
"issue": "11",
"journal-title": "Lancet Respir Med",
"key": "320_CR15",
"unstructured": "Zoufaly A, Poglitsch M, Aberle JH, Hoepler W, Seitz T, Traugott M, et al. Human recombinant soluble ACE2 in severe COVID-19. Lancet Respir Med. 2020;8(11):1154–8. https://doi.org/10.1016/s2213-2600(20)30418-5.",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1128/jvi.00685-21",
"author": "M Ferrari",
"doi-asserted-by": "publisher",
"issue": "19",
"journal-title": "J Virol",
"key": "320_CR16",
"unstructured": "Ferrari M, Mekkaoui L, Ilca FT, Akbar Z, Bughda R, Lamb K, et al. Characterization of a novel ACE2-based therapeutic with enhanced rather than reduced activity against SARS-CoV-2 variants. J Virol. 2021;95(19):e0068521. https://doi.org/10.1128/jvi.00685-21.",
"volume": "95",
"year": "2021"
},
{
"DOI": "10.1038/s41467-021-24013-y",
"author": "Y Higuchi",
"doi-asserted-by": "publisher",
"first-page": "3802",
"issue": "1",
"journal-title": "Nat Commun",
"key": "320_CR17",
"unstructured": "Higuchi Y, Suzuki T, Arimori T, Ikemura N, Mihara E, Kirita Y, et al. Engineered ACE2 receptor therapy overcomes mutational escape of SARS-CoV-2. Nat Commun. 2021;12(1):3802. https://doi.org/10.1038/s41467-021-24013-y.",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1016/j.isci.2021.103670",
"author": "N Iwanaga",
"doi-asserted-by": "publisher",
"issue": "1",
"journal-title": "iScience",
"key": "320_CR18",
"unstructured": "Iwanaga N, Cooper L, Rong L, Maness NJ, Beddingfield B, Qin Z, et al. ACE2-IgG1 fusions with improved in vitro and in vivo activity against SARS-CoV-2. iScience. 2022;25(1):103670. https://doi.org/10.1016/j.isci.2021.103670.",
"volume": "25",
"year": "2022"
},
{
"DOI": "10.1002/jmv.26232",
"author": "B Hu",
"doi-asserted-by": "publisher",
"first-page": "250",
"issue": "1",
"journal-title": "J Med Virol",
"key": "320_CR19",
"unstructured": "Hu B, Huang S, Yin L. The cytokine storm and COVID-19. J Med Virol. 2021;93(1):250–6. https://doi.org/10.1002/jmv.26232.",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1080/22221751.2020.1770129",
"author": "H Han",
"doi-asserted-by": "publisher",
"first-page": "1123",
"issue": "1",
"journal-title": "Emerg Microbes Infect",
"key": "320_CR20",
"unstructured": "Han H, Ma Q, Li C, Liu R, Zhao L, Wang W, et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect. 2020;9(1):1123–30. https://doi.org/10.1080/22221751.2020.1770129.",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1126/science.abb8925",
"author": "JB Moore",
"doi-asserted-by": "publisher",
"first-page": "473",
"issue": "6490",
"journal-title": "Science",
"key": "320_CR21",
"unstructured": "Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science. 2020;368(6490):473–4. https://doi.org/10.1126/science.abb8925.",
"volume": "368",
"year": "2020"
},
{
"DOI": "10.1038/s41591-020-1051-9",
"author": "DM Del Valle",
"doi-asserted-by": "publisher",
"first-page": "1636",
"issue": "10",
"journal-title": "Nat Med",
"key": "320_CR22",
"unstructured": "Del Valle DM, Kim-Schulze S, Huang HH, Beckmann ND, Nirenberg S, Wang B, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26(10):1636–43. https://doi.org/10.1038/s41591-020-1051-9.",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1126/science.abc6027",
"author": "J Hadjadj",
"doi-asserted-by": "publisher",
"first-page": "718",
"issue": "6504",
"journal-title": "Science",
"key": "320_CR23",
"unstructured": "Hadjadj J, Yatim N, Barnabei L, Corneau A, Boussier J, Smith N, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369(6504):718–24. https://doi.org/10.1126/science.abc6027.",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1038/s41577-023-00856-y",
"author": "S Rose-John",
"doi-asserted-by": "publisher",
"first-page": "666",
"issue": "10",
"journal-title": "Nat Rev Immunol",
"key": "320_CR24",
"unstructured": "Rose-John S, Jenkins BJ, Garbers C, Moll JM, Scheller J. Targeting IL-6 trans-signalling: past, present and future prospects. Nat Rev Immunol. 2023;23(10):666–81. https://doi.org/10.1038/s41577-023-00856-y.",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.1016/0092-8674(89)90438-8",
"author": "T Taga",
"doi-asserted-by": "publisher",
"first-page": "573",
"issue": "3",
"journal-title": "Cell",
"key": "320_CR25",
"unstructured": "Taga T, Hibi M, Hirata Y, Yamasaki K, Yasukawa K, Matsuda T, et al. Interleukin-6 triggers the association of its receptor with a possible signal transducer, gp130. Cell. 1989;58(3):573–81. https://doi.org/10.1016/0092-8674(89)90438-8.",
"volume": "58",
"year": "1989"
},
{
"DOI": "10.1016/0092-8674(90)90411-7",
"author": "M Hibi",
"doi-asserted-by": "publisher",
"first-page": "1149",
"issue": "6",
"journal-title": "Cell",
"key": "320_CR26",
"unstructured": "Hibi M, Murakami M, Saito M, Hirano T, Taga T, Kishimoto T. Molecular cloning and expression of an IL-6 signal transducer, gp130. Cell. 1990;63(6):1149–57. https://doi.org/10.1016/0092-8674(90)90411-7.",
"volume": "63",
"year": "1990"
},
{
"DOI": "10.1038/s41392-021-00821-y",
"author": "A Zheng",
"doi-asserted-by": "publisher",
"first-page": "10",
"issue": "1",
"journal-title": "Signal Transduct Target Ther",
"key": "320_CR27",
"unstructured": "Zheng A, Wu L, Ma R, Han P, Huang B, Qiao C, et al. A binding-enhanced but enzymatic activity-eliminated human ACE2 efficiently neutralizes SARS-CoV-2 variants. Signal Transduct Target Ther. 2022;7(1):10. https://doi.org/10.1038/s41392-021-00821-y.",
"volume": "7",
"year": "2022"
},
{
"DOI": "10.1126/science.abc0870",
"author": "KK Chan",
"doi-asserted-by": "publisher",
"first-page": "1261",
"issue": "6508",
"journal-title": "Science",
"key": "320_CR28",
"unstructured": "Chan KK, Dorosky D, Sharma P, Abbasi SA, Dye JM, Kranz DM, et al. Engineering human ACE2 to optimize binding to the spike protein of SARS coronavirus 2. Science. 2020;369(6508):1261–5. https://doi.org/10.1126/science.abc0870.",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2021.12.046",
"doi-asserted-by": "publisher",
"key": "320_CR29",
"unstructured": "Dejnirattisai W, Huo J, Zhou D, Zahradník J, Supasa P, Liu C, et al., SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell, 2022;185(3):467–84.e15. https://doi.org/10.1016/j.cell.2021.12.046."
},
{
"DOI": "10.4161/mabs.22276",
"author": "Q Tan",
"doi-asserted-by": "publisher",
"first-page": "761",
"issue": "6",
"journal-title": "MAbs",
"key": "320_CR30",
"unstructured": "Tan Q, Guo Q, Fang C, Wang C, Li B, Wang H, et al. Characterization and comparison of commercially available TNF receptor 2-Fc fusion protein products. MAbs. 2012;4(6):761–74. https://doi.org/10.4161/mabs.22276.",
"volume": "4",
"year": "2012"
},
{
"DOI": "10.1016/S0021-9258(18)82467-X",
"author": "M van Dam",
"doi-asserted-by": "publisher",
"first-page": "15285",
"issue": "20",
"journal-title": "J Biol Chem",
"key": "320_CR31",
"unstructured": "van Dam M, Müllberg J, Schooltink H, Stoyan T, Brakenhoff JP, Graeve L, et al. Structure-function analysis of interleukin-6 utilizing human/murine chimeric molecules. Involvement of two separate domains in receptor binding. J Biol Chem. 1993;268(20):15285–90.",
"volume": "268",
"year": "1993"
},
{
"DOI": "10.1046/j.1432-1327.2001.01867.x",
"author": "T Jostock",
"doi-asserted-by": "publisher",
"first-page": "160",
"issue": "1",
"journal-title": "Eur J Biochem",
"key": "320_CR32",
"unstructured": "Jostock T, Müllberg J, Ozbek S, Atreya R, Blinn G, Voltz N, et al. Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. Eur J Biochem. 2001;268(1):160–7. https://doi.org/10.1046/j.1432-1327.2001.01867.x.",
"volume": "268",
"year": "2001"
},
{
"DOI": "10.1371/journal.ppat.1011589",
"author": "GR Meehan",
"doi-asserted-by": "publisher",
"issue": "11",
"journal-title": "PLoS Pathog",
"key": "320_CR33",
"unstructured": "Meehan GR, Herder V, Allan J, Huang X, Kerr K, Mendonca DC, et al. Phenotyping the virulence of SARS-CoV-2 variants in hamsters by digital pathology and machine learning. PLoS Pathog. 2023;19(11):e1011589. https://doi.org/10.1371/journal.ppat.1011589.",
"volume": "19",
"year": "2023"
},
{
"DOI": "10.1001/jama.2023.16498",
"author": "J Abbasi",
"doi-asserted-by": "publisher",
"first-page": "900",
"issue": "10",
"journal-title": "JAMA",
"key": "320_CR34",
"unstructured": "Abbasi J. What to Know About EG.5, the Latest SARS-CoV-2 “Variant of Interest.” JAMA. 2023;330(10):900–1. https://doi.org/10.1001/jama.2023.16498.",
"volume": "330",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2031994",
"author": "AC Kalil",
"doi-asserted-by": "publisher",
"first-page": "795",
"issue": "9",
"journal-title": "N Engl J Med",
"key": "320_CR35",
"unstructured": "Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, et al. Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2021;384(9):795–807. https://doi.org/10.1056/NEJMoa2031994.",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.16349",
"author": "CD Spinner",
"doi-asserted-by": "publisher",
"first-page": "1048",
"issue": "11",
"journal-title": "JAMA",
"key": "320_CR36",
"unstructured": "Spinner CD, Gottlieb RL, Criner GJ, Arribas López JR, Cattelan AM, Soriano Viladomiu A, et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA. 2020;324(11):1048–57. https://doi.org/10.1001/jama.2020.16349.",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2116044",
"doi-asserted-by": "publisher",
"key": "320_CR37",
"unstructured": "Jayk Bernal A, Gomes da Silva M M, Musungaie D B, Kovalchuk E, Gonzalez A, Delos Reyes V, et al., Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. N Engl J Med, 2022;386(6):509–20. https://doi.org/10.1056/NEJMoa2116044."
},
{
"DOI": "10.1016/j.tips.2009.11.001",
"author": "M Sauerborn",
"doi-asserted-by": "publisher",
"first-page": "53",
"issue": "2",
"journal-title": "Trends Pharmacol Sci",
"key": "320_CR38",
"unstructured": "Sauerborn M, Brinks V, Jiskoot W, Schellekens H. Immunological mechanism underlying the immune response to recombinant human protein therapeutics. Trends Pharmacol Sci. 2010;31(2):53–9. https://doi.org/10.1016/j.tips.2009.11.001.",
"volume": "31",
"year": "2010"
},
{
"DOI": "10.1146/annurev.immunol.19.1.163",
"author": "M Feldmann",
"doi-asserted-by": "publisher",
"first-page": "163",
"journal-title": "Annu Rev Immunol",
"key": "320_CR39",
"unstructured": "Feldmann M, Maini RN. Anti-TNF alpha therapy of rheumatoid arthritis: what have we learned? Annu Rev Immunol. 2001;19:163–96. https://doi.org/10.1146/annurev.immunol.19.1.163.",
"volume": "19",
"year": "2001"
},
{
"DOI": "10.1164/rccm.201512-2368OC",
"author": "SM Ruwanpura",
"doi-asserted-by": "publisher",
"first-page": "1494",
"issue": "12",
"journal-title": "Am J Respir Crit Care Med",
"key": "320_CR40",
"unstructured": "Ruwanpura SM, McLeod L, Dousha LF, Seow HJ, Alhayyani S, Tate MD, et al. Therapeutic targeting of the IL-6 trans-signaling/mechanistic target of rapamycin complex 1 axis in pulmonary emphysema. Am J Respir Crit Care Med. 2016;194(12):1494–505. https://doi.org/10.1164/rccm.201512-2368OC.",
"volume": "194",
"year": "2016"
},
{
"DOI": "10.1053/j.gastro.2021.02.062",
"author": "S Schreiber",
"doi-asserted-by": "publisher",
"first-page": "2354",
"issue": "7",
"journal-title": "Gastroenterology",
"key": "320_CR41",
"unstructured": "Schreiber S, Aden K, Bernardes JP, Conrad C, Tran F, Höper H, et al. Therapeutic Interleukin-6 Trans-signaling Inhibition by Olamkicept (sgp130Fc) in Patients With Active Inflammatory Bowel Disease. Gastroenterology. 2021;160(7):2354-66.e11. https://doi.org/10.1053/j.gastro.2021.02.062.",
"volume": "160",
"year": "2021"
},
{
"DOI": "10.1016/j.cytox.2021.100058",
"author": "AF Berg",
"doi-asserted-by": "publisher",
"issue": "4",
"journal-title": "Cytokine X",
"key": "320_CR42",
"unstructured": "Berg AF, Ettich J, Weitz HT, Krusche M, Floss DM, Scheller J, et al. Exclusive inhibition of IL-6 trans-signaling by soluble gp130(FlyR)Fc. Cytokine X. 2021;3(4):100058. https://doi.org/10.1016/j.cytox.2021.100058.",
"volume": "3",
"year": "2021"
},
{
"DOI": "10.1073/pnas.0409465102",
"author": "H Hofmann",
"doi-asserted-by": "publisher",
"first-page": "7988",
"issue": "22",
"journal-title": "Proc Natl Acad Sci U S A",
"key": "320_CR43",
"unstructured": "Hofmann H, Pyrc K, van der Hoek L, Geier M, Berkhout B, Pöhlmann S. Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry. Proc Natl Acad Sci U S A. 2005;102(22):7988–93. https://doi.org/10.1073/pnas.0409465102.",
"volume": "102",
"year": "2005"
},
{
"DOI": "10.1038/s41586-022-04532-4",
"author": "S Temmam",
"doi-asserted-by": "publisher",
"first-page": "330",
"issue": "7905",
"journal-title": "Nature",
"key": "320_CR44",
"unstructured": "Temmam S, Vongphayloth K, Baquero E, Munier S, Bonomi M, Regnault B, et al. Bat coronaviruses related to SARS-CoV-2 and infectious for human cells. Nature. 2022;604(7905):330–6. https://doi.org/10.1038/s41586-022-04532-4.",
"volume": "604",
"year": "2022"
},
{
"DOI": "10.1016/j.cell.2025.01.042",
"author": "J Chen",
"doi-asserted-by": "publisher",
"first-page": "1729",
"issue": "6",
"journal-title": "Cell",
"key": "320_CR45",
"unstructured": "Chen J, Zhang W, Li Y, Liu C, Dong T, Chen H, et al. Bat-infecting merbecovirus HKU5-CoV lineage 2 can use human ACE2 as a cell entry receptor. Cell. 2025;188(6):1729-42.e16. https://doi.org/10.1016/j.cell.2025.01.042.",
"volume": "188",
"year": "2025"
},
{
"DOI": "10.1038/nbt0297-142",
"doi-asserted-by": "publisher",
"key": "320_CR46",
"unstructured": "Fischer M, Goldschmitt J, Peschel C, Brakenhoff JP, Kallen K J, Wollmer A, et al. I. A bioactive designer cytokine for human hematopoietic progenitor cell expansion. Nat Biotechnol, 1997;15(2):142–5. https://doi.org/10.1038/nbt0297-142."
}
],
"reference-count": 46,
"references-count": 46,
"relation": {},
"resource": {
"primary": {
"URL": "https://link.springer.com/10.1186/s43556-025-00320-4"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Development of a trispecific fusion protein based on angiotensin-converting enzyme 2, glycoprotein 130, and tumor necrosis factor receptor 2 as a promising therapeutic for COVID-19",
"type": "journal-article",
"update-policy": "https://doi.org/10.1007/springer_crossmark_policy",
"volume": "6"
}