Efficacy of Losartan in Hospitalized Patients With COVID-19–Induced Lung Injury
et al., JAMA Network Open, doi:10.1001/jamanetworkopen.2022.2735, ALPS-IP, NCT04312009, Mar 2022
RCT 205 hospitalized COVID-19 patients showing no significant difference in lung injury (PaO2:FiO2 ratio) at day 7 with losartan treatment, and no difference in clinical outcomes including mortality. Losartan was associated with more adverse events including acute kidney injury and need for vasopressors. Results suggest losartan is not beneficial and may cause harm in this setting.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
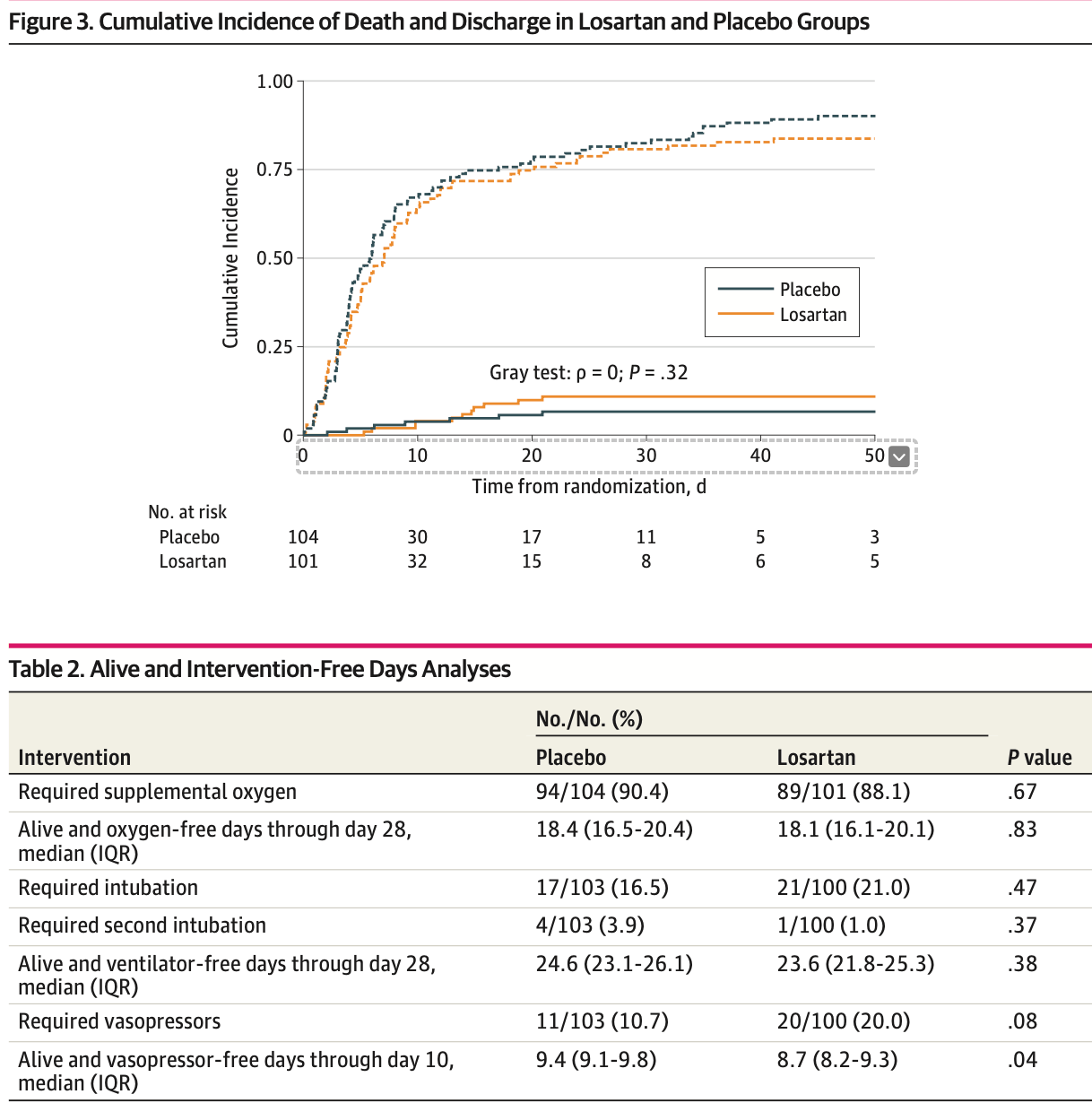
risk of death, 3.0% higher, RR 1.03, p = 1.00, treatment 11 of 101 (10.9%), control 11 of 104 (10.6%), day 90.
|
|
risk of death, 25.9% higher, RR 1.26, p = 0.64, treatment 11 of 101 (10.9%), control 9 of 104 (8.7%), day 28.
|
|
risk of mechanical ventilation, 27.2% higher, RR 1.27, p = 0.47, treatment 21 of 100 (21.0%), control 17 of 103 (16.5%).
|
|
risk of oxygen therapy, 2.5% lower, RR 0.97, p = 0.66, treatment 89 of 101 (88.1%), control 94 of 104 (90.4%), NNT 44.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Puskarich et al., 16 Mar 2022, Double Blind Randomized Controlled Trial, placebo-controlled, USA, peer-reviewed, 36 authors, study period April 2020 - February 2021, trial NCT04312009 (history) (ALPS-IP).
Efficacy of Losartan in Hospitalized Patients With COVID-19–Induced Lung Injury
JAMA Network Open, doi:10.1001/jamanetworkopen.2022.2735
IMPORTANCE SARS-CoV-2 viral entry may disrupt angiotensin II (AII) homeostasis, contributing to COVID-19 induced lung injury. AII type 1 receptor blockade mitigates lung injury in preclinical models, although data in humans with COVID-19 remain mixed. OBJECTIVE To test the efficacy of losartan to reduce lung injury in hospitalized patients with COVID-19. DESIGN, SETTING, AND PARTICIPANTS This blinded, placebo-controlled randomized clinical trial was conducted in 13 hospitals in the United States from April 2020 to February 2021. Hospitalized patients with COVID-19 and a respiratory sequential organ failure assessment score of at least 1 and not already using a renin-angiotensin-aldosterone system (RAAS) inhibitor were eligible for participation. Data were analyzed from April 19 to August 24, 2021. INTERVENTIONS Losartan 50 mg orally twice daily vs equivalent placebo for 10 days or until hospital discharge. MAIN OUTCOMES AND MEASURES The primary outcome was the imputed arterial partial pressure of oxygen to fraction of inspired oxygen (PaO 2 :FiO 2 ) ratio at 7 days. Secondary outcomes included ordinal COVID-19 severity; days without supplemental O 2 , ventilation, or vasopressors; and mortality. Losartan pharmacokinetics and RAAS components (AII, angiotensin-[1-7] and angiotensinconverting enzymes 1 and 2)] were measured in a subgroup of participants. RESULTS A total of 205 participants (mean [SD] age, 55.2 [15.7] years; 123 [60.0%] men) were randomized, with 101 participants assigned to losartan and 104 participants assigned to placebo. Compared with placebo, losartan did not significantly affect PaO 2 :FiO 2 ratio at 7 days (difference, -24.8 [95%, -55.6 to 6.1]; P = .12). Compared with placebo, losartan did not improve any secondary clinical outcomes and led to fewer vasopressor-free days than placebo (median [IQR], 9.4 [9.1-9.8] vasopressor-free days vs 8.7 [8.2-9.3] vasopressor-free days).
CONCLUSIONS AND RELEVANCE This randomized clinical trial found that initiation of orally administered losartan to hospitalized patients with COVID-19 and acute lung injury did not improve PaO 2 :FiO 2 ratio at 7 days. These data may have implications for ongoing clinical trials.
Author Contributions: Dr Puskarich had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Puskarich, Ingraham, Wacker, South, Murray, Biros, Chipman, Schacker, Koopmeiners, Tignanelli. Acquisition, analysis, or interpretation of data: Puskarich, Ingraham, Merck, Driver, Wacker, Black, Jones, Fletcher, South, Murray, Lewandowski, Farhat, Benoit, Biros, Cherabuddi, Guirgis, Voelker, Koopmeiners, Tignanelli. Drafting of the manuscript: Puskarich, Ingraham, Fletcher, Murray, Schacker, Koopmeiners, Tignanelli. Funding/Support: The study was primarily funded by grant No. INV-017069 from the Bill and Melinda Gates Foundation. Dr South was supported by the NIH NHLBI (grants No. K23HL148394, L40HL148910, and R01HL146818). Pharmacokinetic data analyses were supported by grant No. R01AI124965 from the NIH (Dr Fletcher).
Critical revision of the manuscript for important intellectual content
Role of the Funder/Sponsor: The funder contributed to the study design, but had no role in the conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. Additional Contributions: Lee Winchester, BS, and Timothy Mykris, BS (Antiviral Pharmacology Laboratory, University of Nebraska Medical Center), assisted with quantitation of losartan and carboxylosartan..
References
Beigel, Tomashek, Dodd, ACTT-1 Study Group Members. Remdesivir for the treatment of COVID-19-final report, N Engl J Med, doi:10.1056/NEJMoa2007764
Brosnihan, Chappell, Measurement of angiotensin peptides: HPLC-RIA, Methods Mol Biol, doi:10.1007/978-1-4939-6625-7_7
Brun-Buisson, Richard, Mercat, Thiébaut, Brochard, 1N1v 2009 Registry Group. Early corticosteroids in severe influenza A/H1N1 pneumonia and acute respiratory distress syndrome, Am J Respir Crit Care Med, doi:10.1164/rccm.201101-0135OC
Caldeira, Alves, Melo, Angiotensin-converting enzyme inhibitors and angiotensinreceptor blockers and the risk of COVID-19 infection or severe disease: systematic review and meta-analysis, Int J Cardiol Heart Vasc, doi:10.1016/j.ijcha.2020.100627
Cohen, Hanff, William, Continuation versus discontinuation of renin-angiotensin system inhibitors in patients admitted to hospital with COVID-19: a prospective, randomised, open-label trial, Lancet Respir Med, doi:10.1016/S2213-2600(20)30558-0
Cohen, Mcgowan, Jensen, Rigdon, South, Evaluating sources of bias in observational studies of angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker use during COVID-19: beyond confounding, J Hypertens, doi:10.1097/HJH.0000000000002706
Duarte, Pelorosso, Nicolosi, Telmisartan for treatment of Covid-19 patients: an open randomized clinical trial-preliminary report, doi:10.1101/2020.08.04.20167205
Ferreira, Bota, Bross, Mélot, Vincent, Serial evaluation of the SOFA score to predict outcome in critically ill patients, JAMA, doi:https://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.286.14.1754&utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jamanetworkopen.2022.2735
Files, Gibbs, Schaich, A pilot study to assess the circulating renin-angiotensin system in COVID-19 acute respiratory failure, Am J Physiol Lung Cell Mol Physiol, doi:10.1152/ajplung.00129.2021
Gadrey, Lau, Clay, Imputation of partial pressures of arterial oxygen using oximetry and its impact on sepsis diagnosis, Physiol Meas, doi:10.1088/1361-6579/ab5154
Harris, Taylor, Minor, The REDCap consortium: building an international community of software platform partners, J Biomed Inform, doi:10.1016/j.jbi.2019.103208
Hasan, Kow, Hadi, Zaidi, Merchant, Mortality and disease severity among COVID-19 patients receiving renin-angiotensin system inhibitors: a systematic review and meta-analysis, Am J Cardiovasc Drugs, doi:10.1007/s40256-020-00439-5
Henry, Benoit, Lippi, Benoit, Letter to the Editor-circulating plasma levels of angiotensin II and aldosterone in patients with coronavirus disease 2019 (COVID-19): a preliminary report, Prog Cardiovasc Dis, doi:10.1016/j.pcad.2020.07.006
Imai, Kuba, Penninger, The discovery of angiotensin-converting enzyme 2 and its role in acute lung injury in mice, Exp Physiol, doi:10.1113/expphysiol.2007.040048
Imai, Kuba, Rao, Angiotensin-converting enzyme 2 protects from severe acute lung failure, Nature, doi:10.1038/nature03712
Ingraham, Barakat, Reilkoff, Understanding the renin-angiotensin-aldosterone-SARS-CoV axis: a comprehensive review, Eur Respir J, doi:10.1183/13993003.00912-2020
Ingraham, Lotfi-Emran, Thielen, Immunomodulation in COVID-19, Lancet Respir Med, doi:10.1016/S2213-2600(20)30226-5
Kellum, Lameire, Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (part 1), Crit Care, doi:10.1186/cc11454
Lopes, Macedo, De, Silva, Effect of discontinuing vs continuing angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on days alive and out of the hospital in patients admitted with COVID-19: a randomized clinical trial, JAMA, doi:https://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.2020.25864&utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jamanetworkopen.2022.2735
Lusczek, Ingraham, Karam, Characterizing COVID-19 clinical phenotypes and associated comorbidities and complication profiles, PLoS One, doi:10.1371/journal.pone.0248956
Moss, Huang, Brower, Early neuromuscular blockade in the acute respiratory distress syndrome, N Engl J Med, doi:10.1056/NEJMoa1901686
Munafo, Christen, Nussberger, Drug concentration response relationships in normal volunteers after oral administration of losartan, an angiotensin II receptor antagonist, Clin Pharmacol Ther, doi:10.1038/clpt.1992.56
Naveed, Elshafeey, Al-Ali, The interplay between the immune system, the renin-angiotensinaldosterone system (RAAS), and RAAS inhibitors may modulate the outcome of COVID-19: a systematic review, J Clin Pharmacol, doi:10.1002/jcph.1852
Osman, Melenotte, Brouqui, Expression of ACE2, soluble ACE2, angiotensin I, angiotensin II and angiotensin-(1-7) is modulated in COVID-19 patients, Front Immunol, doi:10.3389/fimmu.2021.625732
Pandharipande, Shintani, Hagerman, Derivation and validation of SPO 2 /FiO 2 ratio to impute for PaO 2 /FiO 2 ratio in the respiratory component of the Sequential Organ Failure Assessment score, Crit Care Med, doi:10.1097/CCM.0b013e31819cefa9
Perrotta, Corbi, Mazzeo, COVID-19 and the elderly: insights into pathogenesis and clinical decisionmaking, Aging Clin Exp Res, doi:10.1007/s40520-020-01631-y
Polverino, Stern, Ruocco, ItaliCO study group. Comorbidities, cardiovascular therapies, and COVID-19 mortality: a nationwide, Italian Observational Study (ItaliCO), Front Cardiovasc Med, doi:10.3389/fcvm.2020.585866
Puskarich, Cummins, Ingraham, Effect of losartan on symptomatic outpatients with COVID-19: a randomized clinical trial, doi:10.2139/ssrn.3787463
Quispe-Laime, Bracco, Barberio, H1N1 influenza A virus-associated acute lung injury: response to combination oseltamivir and prolonged corticosteroid treatment, Intensive Care Med, doi:10.1007/s00134-009-1727-6
Riscili, Anderson, Prescott, An assessment of H1N1 influenza-associated acute respiratory distress syndrome severity after adjustment for treatment characteristics, PLoS One, doi:10.1371/journal.pone.0018166
Rouette, Suissa, Azoulay, Re: association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19, Epidemiology, doi:10.1097/EDE.0000000000001250
Semenzato, Botton, Drouin, Antihypertensive drugs and COVID-19 risk: a cohort study of 2 million hypertensive patients, Hypertension, doi:10.1161/HYPERTENSIONAHA.120.16314
Sica, Gehr, Ghosh, Clinical pharmacokinetics of losartan, Clin Pharmacokinet, doi:10.2165/00003088-200544080-00003
South, Diz, Chappell, COVID-19, ACE2, and the cardiovascular consequences, Am J Physiol Heart Circ Physiol, doi:10.1152/ajpheart.00217.2020
South, Nixon, Chappell, Association between preterm birth and the renin-angiotensin system in adolescence: influence of sex and obesity, J Hypertens, doi:10.1097/HJH.0000000000001801
Sparks, South, Badley, Severe acute respiratory syndrome coronavirus 2, COVID-19, and the renin-angiotensin system: pressing needs and best research practices, Hypertension, doi:10.1161/HYPERTENSIONAHA.120.15948
Worldometer, COVID-19 coronavirus pandemic
Zou, Yan, Shu, Angiotensin-converting enzyme 2 protects from lethal avian influenza A H5N1 infections, Nat Commun, doi:10.1038/ncomms4594
DOI record:
{
"DOI": "10.1001/jamanetworkopen.2022.2735",
"ISSN": [
"2574-3805"
],
"URL": "http://dx.doi.org/10.1001/jamanetworkopen.2022.2735",
"author": [
{
"affiliation": [
{
"name": "Department of Emergency Medicine, University of Minnesota, Minneapolis"
},
{
"name": "Department of Emergency Medicine, Hennepin County Medical Center, Minneapolis, Minnesota"
}
],
"family": "Puskarich",
"given": "Michael A.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Division of Pulmonary, Allergy, Critical Care and Sleep Medicine, Department of Medicine, University of Minnesota, Minneapolis"
}
],
"family": "Ingraham",
"given": "Nicholas E.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Emergency Medicine, University of Florida College of Medicine, Gainesville"
}
],
"family": "Merck",
"given": "Lisa H.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Emergency Medicine, Hennepin County Medical Center, Minneapolis, Minnesota"
}
],
"family": "Driver",
"given": "Brian E.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Pulmonary, Allergy, Critical Care and Sleep Medicine, Department of Medicine, University of Minnesota, Minneapolis"
}
],
"family": "Wacker",
"given": "David A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Emergency Medicine, University of Florida College of Medicine, Jacksonville"
}
],
"family": "Black",
"given": "Lauren Page",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Emergency Medicine, University of Mississippi Medical Center, Jackson"
}
],
"family": "Jones",
"given": "Alan E.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Center for Drug Discovery, University of Nebraska Medical Center, Omaha"
}
],
"family": "Fletcher",
"given": "Courtney V.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Section of Nephrology, Department of Pediatrics, Wake Forest School of Medicine and Brenner Children's Hospital, Winston Salem, North Carolina"
},
{
"name": "Division of Public Health Sciences, Department of Epidemiology and Prevention, Wake Forest School of Medicine, Winston Salem, North Carolina"
},
{
"name": "Department of Surgery-Hypertension and Vascular Research, Wake Forest School of Medicine, Winston Salem, North Carolina"
}
],
"family": "South",
"given": "Andrew M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biostatistics, School of Public Health, University of Minnesota, Minneapolis"
}
],
"family": "Murray",
"given": "Thomas A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Emergency Medicine, Henry Ford Hospital, Wayne State University, Detroit, Michigan"
}
],
"family": "Lewandowski",
"given": "Christopher",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Surgery, North Memorial Medical Center, Minneapolis, Minnesota"
}
],
"family": "Farhat",
"given": "Joseph",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Emergency Medicine, University of Cincinnati, Cincinnati, Ohio"
}
],
"family": "Benoit",
"given": "Justin L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Emergency Medicine, University of Minnesota, Minneapolis"
}
],
"family": "Biros",
"given": "Michelle H.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Emergency Medicine, University of Florida College of Medicine, Gainesville"
}
],
"family": "Cherabuddi",
"given": "Kartik",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Surgery, University of Minnesota, Minneapolis"
}
],
"family": "Chipman",
"given": "Jeffrey G.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Disease, Department of Medicine, University of Minnesota, Minneapolis"
}
],
"family": "Schacker",
"given": "Timothy W.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Emergency Medicine, University of Florida College of Medicine, Jacksonville"
}
],
"family": "Guirgis",
"given": "Faheem W.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biostatistics, School of Public Health, University of Minnesota, Minneapolis"
}
],
"family": "Voelker",
"given": "Helen T.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biostatistics, School of Public Health, University of Minnesota, Minneapolis"
}
],
"family": "Koopmeiners",
"given": "Joseph S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Surgery, University of Minnesota, Minneapolis"
}
],
"family": "Tignanelli",
"given": "Christopher J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Angiotensin Receptor Blocker Based Lung Protective Strategies for Inpatients With COVID-19 (ALPS-IP) Investigators"
}
],
"family": "Nelson",
"given": "Andrew C",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Angiotensin Receptor Blocker Based Lung Protective Strategies for Inpatients With COVID-19 (ALPS-IP) Investigators"
}
],
"family": "Hall",
"given": "Alex",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Angiotensin Receptor Blocker Based Lung Protective Strategies for Inpatients With COVID-19 (ALPS-IP) Investigators"
}
],
"family": "Wright",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Angiotensin Receptor Blocker Based Lung Protective Strategies for Inpatients With COVID-19 (ALPS-IP) Investigators"
}
],
"family": "Reilkoff",
"given": "Ronald A",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Angiotensin Receptor Blocker Based Lung Protective Strategies for Inpatients With COVID-19 (ALPS-IP) Investigators"
}
],
"family": "Bold",
"given": "Tyler",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Angiotensin Receptor Blocker Based Lung Protective Strategies for Inpatients With COVID-19 (ALPS-IP) Investigators"
}
],
"family": "Beckman",
"given": "Kenneth",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Angiotensin Receptor Blocker Based Lung Protective Strategies for Inpatients With COVID-19 (ALPS-IP) Investigators"
}
],
"family": "Langlois",
"given": "Ryan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Angiotensin Receptor Blocker Based Lung Protective Strategies for Inpatients With COVID-19 (ALPS-IP) Investigators"
}
],
"family": "Aliota",
"given": "Matthew T",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Angiotensin Receptor Blocker Based Lung Protective Strategies for Inpatients With COVID-19 (ALPS-IP) Investigators"
}
],
"family": "Galbriath",
"given": "James",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Angiotensin Receptor Blocker Based Lung Protective Strategies for Inpatients With COVID-19 (ALPS-IP) Investigators"
}
],
"family": "Beyer",
"given": "Margaret",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Angiotensin Receptor Blocker Based Lung Protective Strategies for Inpatients With COVID-19 (ALPS-IP) Investigators"
}
],
"family": "Salmen",
"given": "Chas",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Angiotensin Receptor Blocker Based Lung Protective Strategies for Inpatients With COVID-19 (ALPS-IP) Investigators"
}
],
"family": "Byrne",
"given": "Dana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Angiotensin Receptor Blocker Based Lung Protective Strategies for Inpatients With COVID-19 (ALPS-IP) Investigators"
}
],
"family": "Roberts",
"given": "Brian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the Angiotensin Receptor Blocker Based Lung Protective Strategies for Inpatients With COVID-19 (ALPS-IP) Investigators"
}
],
"family": "James",
"given": "Nastasia",
"sequence": "additional"
},
{
"affiliation": [],
"name": "Angiotensin Receptor Blocker Based Lung Protective Strategies for Inpatients With COVID-19 (ALPS-IP) Investigators",
"sequence": "additional"
}
],
"container-title": "JAMA Network Open",
"container-title-short": "JAMA Netw Open",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
3,
16
]
],
"date-time": "2022-03-16T15:02:01Z",
"timestamp": 1647442921000
},
"deposited": {
"date-parts": [
[
2022,
5,
17
]
],
"date-time": "2022-05-17T16:45:19Z",
"timestamp": 1652805919000
},
"indexed": {
"date-parts": [
[
2024,
5,
22
]
],
"date-time": "2024-05-22T05:14:59Z",
"timestamp": 1716354899998
},
"is-referenced-by-count": 42,
"issue": "3",
"issued": {
"date-parts": [
[
2022,
3,
16
]
]
},
"journal-issue": {
"issue": "3",
"published-print": {
"date-parts": [
[
2022,
3,
1
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://jamanetwork.com/journals/jamanetworkopen/articlepdf/2790162/puskarich_2022_oi_220109_1652198565.7699.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "10",
"original-title": [],
"page": "e222735",
"prefix": "10.1001",
"published": {
"date-parts": [
[
2022,
3,
16
]
]
},
"published-online": {
"date-parts": [
[
2022,
3,
16
]
]
},
"publisher": "American Medical Association (AMA)",
"reference": [
{
"DOI": "10.1183/13993003.00912-2020",
"article-title": "Understanding the renin-angiotensin-aldosterone–SARS-CoV axis: a comprehensive review.",
"author": "Ingraham",
"doi-asserted-by": "publisher",
"issue": "1",
"journal-title": "Eur Respir J",
"key": "zoi220109r2",
"volume": "56",
"year": "2020"
},
{
"DOI": "10.1016/S2213-2600(20)30226-5",
"article-title": "Immunomodulation in COVID-19.",
"author": "Ingraham",
"doi-asserted-by": "publisher",
"first-page": "544",
"issue": "6",
"journal-title": "Lancet Respir Med",
"key": "zoi220109r3",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1152/ajpheart.00217.2020",
"article-title": "COVID-19, ACE2, and the cardiovascular consequences.",
"author": "South",
"doi-asserted-by": "publisher",
"first-page": "H1084",
"issue": "5",
"journal-title": "Am J Physiol Heart Circ Physiol",
"key": "zoi220109r4",
"volume": "318",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2021.625732",
"article-title": "Expression of ACE2, soluble ACE2, angiotensin I, angiotensin II and angiotensin-(1-7) is modulated in COVID-19 patients.",
"author": "Osman",
"doi-asserted-by": "publisher",
"journal-title": "Front Immunol",
"key": "zoi220109r5",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1152/ajplung.00129.2021",
"article-title": "A pilot study to assess the circulating renin-angiotensin system in COVID-19 acute respiratory failure.",
"author": "Files",
"doi-asserted-by": "publisher",
"first-page": "L213",
"issue": "1",
"journal-title": "Am J Physiol Lung Cell Mol Physiol",
"key": "zoi220109r6",
"volume": "321",
"year": "2021"
},
{
"DOI": "10.1016/j.pcad.2020.07.006",
"article-title": "Letter to the Editor—circulating plasma levels of angiotensin II and aldosterone in patients with coronavirus disease 2019 (COVID-19): a preliminary report.",
"author": "Henry",
"doi-asserted-by": "publisher",
"first-page": "702",
"issue": "5",
"journal-title": "Prog Cardiovasc Dis",
"key": "zoi220109r7",
"volume": "63",
"year": "2020"
},
{
"DOI": "10.1038/nature03712",
"article-title": "Angiotensin-converting enzyme 2 protects from severe acute lung failure.",
"author": "Imai",
"doi-asserted-by": "publisher",
"first-page": "112",
"issue": "7047",
"journal-title": "Nature",
"key": "zoi220109r8",
"volume": "436",
"year": "2005"
},
{
"DOI": "10.1113/expphysiol.2007.040048",
"article-title": "The discovery of angiotensin-converting enzyme 2 and its role in acute lung injury in mice.",
"author": "Imai",
"doi-asserted-by": "publisher",
"first-page": "543",
"issue": "5",
"journal-title": "Exp Physiol",
"key": "zoi220109r9",
"volume": "93",
"year": "2008"
},
{
"DOI": "10.1038/ncomms4594",
"article-title": "Angiotensin-converting enzyme 2 protects from lethal avian influenza A H5N1 infections.",
"author": "Zou",
"doi-asserted-by": "publisher",
"first-page": "3594",
"journal-title": "Nat Commun",
"key": "zoi220109r10",
"volume": "5",
"year": "2014"
},
{
"DOI": "10.1002/jcph.v61.8",
"article-title": "The interplay between the immune system, the renin-angiotensin-aldosterone system (RAAS), and RAAS inhibitors may modulate the outcome of COVID-19: a systematic review.",
"author": "Naveed",
"doi-asserted-by": "publisher",
"first-page": "987",
"issue": "8",
"journal-title": "J Clin Pharmacol",
"key": "zoi220109r11",
"volume": "61",
"year": "2021"
},
{
"DOI": "10.1097/EDE.0000000000001250",
"article-title": "Re: association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19.",
"author": "Rouette",
"doi-asserted-by": "publisher",
"first-page": "e52",
"issue": "6",
"journal-title": "Epidemiology",
"key": "zoi220109r12",
"volume": "31",
"year": "2020"
},
{
"DOI": "10.1007/s40256-020-00439-5",
"article-title": "Mortality and disease severity among COVID-19 patients receiving renin-angiotensin system inhibitors: a systematic review and meta-analysis.",
"author": "Hasan",
"doi-asserted-by": "publisher",
"first-page": "571",
"issue": "6",
"journal-title": "Am J Cardiovasc Drugs",
"key": "zoi220109r13",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1016/j.ijcha.2020.100627",
"article-title": "Angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers and the risk of COVID-19 infection or severe disease: systematic review and meta-analysis.",
"author": "Caldeira",
"doi-asserted-by": "publisher",
"journal-title": "Int J Cardiol Heart Vasc",
"key": "zoi220109r14",
"volume": "31",
"year": "2020"
},
{
"DOI": "10.1016/j.vph.2020.106805",
"article-title": "RAAS inhibitors are not associated with mortality in COVID-19 patients: findings from an observational multicenter study in Italy and a meta-analysis of 19 studies.",
"author": "COVID-19 RISk and Treatments (CORIST) Collaboration",
"doi-asserted-by": "publisher",
"journal-title": "Vascul Pharmacol",
"key": "zoi220109r15",
"volume": "135",
"year": "2020"
},
{
"DOI": "10.3389/fcvm.2020.585866",
"article-title": "Comorbidities, cardiovascular therapies, and COVID-19 mortality: a nationwide, Italian Observational Study (ItaliCO).",
"author": "Polverino",
"doi-asserted-by": "publisher",
"journal-title": "Front Cardiovasc Med",
"key": "zoi220109r16",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1161/HYPERTENSIONAHA.120.16314",
"article-title": "Antihypertensive drugs and COVID-19 risk: a cohort study of 2 million hypertensive patients.",
"author": "Semenzato",
"doi-asserted-by": "publisher",
"first-page": "833",
"issue": "3",
"journal-title": "Hypertension",
"key": "zoi220109r17",
"volume": "77",
"year": "2021"
},
{
"DOI": "10.1001/jama.286.14.1754",
"article-title": "Serial evaluation of the SOFA score to predict outcome in critically ill patients.",
"author": "Ferreira",
"doi-asserted-by": "publisher",
"first-page": "1754",
"issue": "14",
"journal-title": "JAMA",
"key": "zoi220109r19",
"volume": "286",
"year": "2001"
},
{
"DOI": "10.1097/CCM.0b013e31819cefa9",
"article-title": "Derivation and validation of Spo2/Fio2 ratio to impute for Pao2/Fio2 ratio in the respiratory component of the Sequential Organ Failure Assessment score.",
"author": "Pandharipande",
"doi-asserted-by": "publisher",
"first-page": "1317",
"issue": "4",
"journal-title": "Crit Care Med",
"key": "zoi220109r20",
"volume": "37",
"year": "2009"
},
{
"DOI": "10.1016/j.jbi.2019.103208",
"article-title": "The REDCap consortium: building an international community of software platform partners.",
"author": "Harris",
"doi-asserted-by": "publisher",
"journal-title": "J Biomed Inform",
"key": "zoi220109r21",
"volume": "95",
"year": "2019"
},
{
"DOI": "10.2165/00003088-200544080-00003",
"article-title": "Clinical pharmacokinetics of losartan.",
"author": "Sica",
"doi-asserted-by": "publisher",
"first-page": "797",
"issue": "8",
"journal-title": "Clin Pharmacokinet",
"key": "zoi220109r22",
"volume": "44",
"year": "2005"
},
{
"DOI": "10.1088/1361-6579/ab5154",
"article-title": "Imputation of partial pressures of arterial oxygen using oximetry and its impact on sepsis diagnosis.",
"author": "Gadrey",
"doi-asserted-by": "publisher",
"issue": "11",
"journal-title": "Physiol Meas",
"key": "zoi220109r23",
"volume": "40",
"year": "2019"
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the treatment of COVID-19—final report.",
"author": "Beigel",
"doi-asserted-by": "publisher",
"first-page": "1813",
"issue": "19",
"journal-title": "N Engl J Med",
"key": "zoi220109r24",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1186/cc11454",
"article-title": "Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (part 1).",
"author": "Kellum",
"doi-asserted-by": "publisher",
"first-page": "204",
"issue": "1",
"journal-title": "Crit Care",
"key": "zoi220109r25",
"volume": "17",
"year": "2013"
},
{
"DOI": "10.1161/HYPERTENSIONAHA.120.15948",
"article-title": "Severe acute respiratory syndrome coronavirus 2, COVID-19, and the renin-angiotensin system: pressing needs and best research practices.",
"author": "Sparks",
"doi-asserted-by": "publisher",
"first-page": "1350",
"issue": "5",
"journal-title": "Hypertension",
"key": "zoi220109r26",
"volume": "76",
"year": "2020"
},
{
"DOI": "10.1097/HJH.0000000000001801",
"article-title": "Association between preterm birth and the renin-angiotensin system in adolescence: influence of sex and obesity.",
"author": "South",
"doi-asserted-by": "publisher",
"first-page": "2092",
"issue": "10",
"journal-title": "J Hypertens",
"key": "zoi220109r27",
"volume": "36",
"year": "2018"
},
{
"DOI": "10.1007/978-1-4939-6625-7",
"article-title": "Measurement of angiotensin peptides: HPLC-RIA.",
"author": "Brosnihan",
"doi-asserted-by": "publisher",
"first-page": "81",
"journal-title": "Methods Mol Biol",
"key": "zoi220109r28",
"volume": "1527",
"year": "2017"
},
{
"DOI": "10.1056/NEJMoa1901686",
"article-title": "Early neuromuscular blockade in the acute respiratory distress syndrome.",
"author": "Moss",
"doi-asserted-by": "publisher",
"first-page": "1997",
"issue": "21",
"journal-title": "N Engl J Med",
"key": "zoi220109r30",
"volume": "380",
"year": "2019"
},
{
"DOI": "10.1371/journal.pone.0018166",
"article-title": "An assessment of H1N1 influenza-associated acute respiratory distress syndrome severity after adjustment for treatment characteristics.",
"author": "Riscili",
"doi-asserted-by": "publisher",
"issue": "3",
"journal-title": "PLoS One",
"key": "zoi220109r31",
"volume": "6",
"year": "2011"
},
{
"DOI": "10.1164/rccm.201101-0135OC",
"article-title": "Early corticosteroids in severe influenza A/H1N1 pneumonia and acute respiratory distress syndrome.",
"author": "Brun-Buisson",
"doi-asserted-by": "publisher",
"first-page": "1200",
"issue": "9",
"journal-title": "Am J Respir Crit Care Med",
"key": "zoi220109r32",
"volume": "183",
"year": "2011"
},
{
"DOI": "10.1007/s00134-009-1727-6",
"article-title": "H1N1 influenza A virus-associated acute lung injury: response to combination oseltamivir and prolonged corticosteroid treatment.",
"author": "Quispe-Laime",
"doi-asserted-by": "publisher",
"first-page": "33",
"issue": "1",
"journal-title": "Intensive Care Med",
"key": "zoi220109r33",
"volume": "36",
"year": "2010"
},
{
"DOI": "10.1371/journal.pone.0248956",
"article-title": "Characterizing COVID-19 clinical phenotypes and associated comorbidities and complication profiles.",
"author": "Lusczek",
"doi-asserted-by": "publisher",
"issue": "3",
"journal-title": "PLoS One",
"key": "zoi220109r34",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1007/s40520-020-01631-y",
"article-title": "COVID-19 and the elderly: insights into pathogenesis and clinical decision-making.",
"author": "Perrotta",
"doi-asserted-by": "publisher",
"first-page": "1599",
"issue": "8",
"journal-title": "Aging Clin Exp Res",
"key": "zoi220109r35",
"volume": "32",
"year": "2020"
},
{
"DOI": "10.1038/clpt.1992.56",
"article-title": "Drug concentration response relationships in normal volunteers after oral administration of losartan, an angiotensin II receptor antagonist.",
"author": "Munafo",
"doi-asserted-by": "publisher",
"first-page": "513",
"issue": "5",
"journal-title": "Clin Pharmacol Ther",
"key": "zoi220109r36",
"volume": "51",
"year": "1992"
},
{
"DOI": "10.1097/HJH.0000000000002706",
"article-title": "Evaluating sources of bias in observational studies of angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker use during COVID-19: beyond confounding.",
"author": "Cohen",
"doi-asserted-by": "publisher",
"first-page": "795",
"issue": "4",
"journal-title": "J Hypertens",
"key": "zoi220109r37",
"volume": "39",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.25864",
"article-title": "Effect of discontinuing vs continuing angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on days alive and out of the hospital in patients admitted with COVID-19: a randomized clinical trial.",
"author": "Lopes",
"doi-asserted-by": "publisher",
"first-page": "254",
"issue": "3",
"journal-title": "JAMA",
"key": "zoi220109r39",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(20)30558-0",
"article-title": "Continuation versus discontinuation of renin-angiotensin system inhibitors in patients admitted to hospital with COVID-19: a prospective, randomised, open-label trial.",
"author": "Cohen",
"doi-asserted-by": "publisher",
"first-page": "275",
"issue": "3",
"journal-title": "Lancet Respir Med",
"key": "zoi220109r40",
"volume": "9",
"year": "2021"
},
{
"key": "zoi220109r1",
"unstructured": "Worldometer. COVID-19 coronavirus pandemic. Accessed January 5, 2021. https://www.worldometers.info/coronavirus/"
},
{
"key": "zoi220109r18",
"unstructured": "Centers for Disease Control and Prevention. Symptoms of COVID-19. Updated January 4, 2021. Accessed January 5, 2021. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html"
},
{
"DOI": "10.2139/ssrn.3787463",
"doi-asserted-by": "crossref",
"key": "zoi220109r29",
"unstructured": "Puskarich? M, Cummins? NW, Ingraham? N, . Effect of losartan on symptomatic outpatients with COVID-19: a randomized clinical trial.? SSRN. Preprint posted online February 17, 2021. doi:10.2139/ssrn.3787463"
},
{
"DOI": "10.1101/2020.08.04.20167205",
"doi-asserted-by": "crossref",
"key": "zoi220109r38",
"unstructured": "Duarte? M, Pelorosso? FG, Nicolosi? L, ? Telmisartan for treatment of Covid-19 patients: an open randomized clinical trial—preliminary report.? medRxiv. Preprint posted online August 13, 2020. doi:10.1101/2020.08.04.20167205"
}
],
"reference-count": 40,
"references-count": 40,
"relation": {
"has-preprint": [
{
"asserted-by": "object",
"id": "10.1101/2021.08.25.21262623",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2790162"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [
"A Randomized Clinical Trial"
],
"title": "Efficacy of Losartan in Hospitalized Patients With COVID-19–Induced Lung Injury",
"type": "journal-article",
"volume": "5"
}