COVID-19 in Relation to Chronic Antihistamine Prescription
et al., Microorganisms, doi:10.3390/microorganisms12122589, Dec 2024
11th treatment shown to reduce risk in
December 2020, now with p = 0.000052 from 17 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
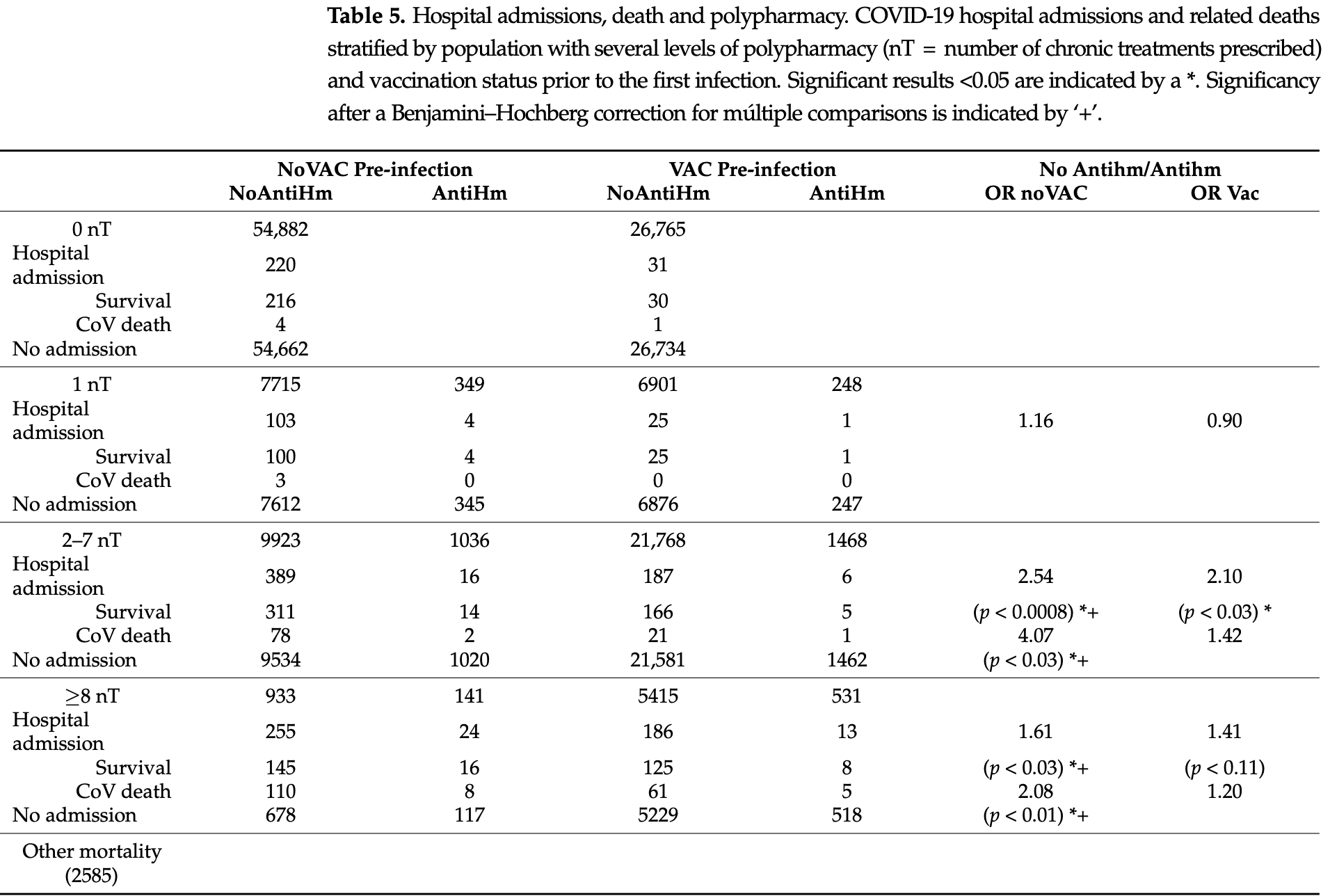
Retrospective 140,681 patients in Spain showing lower COVID-19 infection, hospitalization, and mortality with chronic antihistamine treatment, with a greater protective effect for patients taking 2-7 chronic treatments. Results are provided for all antihistamines, authors do not specify the antihistamines used or provide separate results for H1RAs and H2RAs.
Puigdellívol-Sánchez et al., 13 Dec 2024, retrospective, Spain, peer-reviewed, 8 authors.
Contact: apuigdellivol@cst.cat (corresponding author), mjuanes@cst.cat, acalceronv@cst.cat, helenalosa@uic.es, rvalls@cst.cat, clozano@cst.cat, mgonzalezs@cst.cat, jvidal.cc.ics@gencat.cat.
COVID-19 in Relation to Chronic Antihistamine Prescription
Microorganisms, doi:10.3390/microorganisms12122589
No hospitalizations or deaths occurred in residents with the COVID-19 infection, treated with antihistamines and azithromycin, of two external nursing homes during the first wave. We assessed whether patients receiving chronic antihistamines in our institution showed better clinical evolution. COVID-19 admissions and related deaths in the public Hospital of Terrassa (n = 1461) during the pandemic period (11 March 2020-5 May 2023) and cases (n = 32,888) during the period of full suspicion diagnosis (1 June 2020-23 March 2022) were referred to as the number of chronic treatments (nT) including or not including antihistamines (AntiHm or NOAntiHm), and their vaccination status before the first infection (VAC or NoVAC) in our assigned population (n = 140,681 at March 2020) was recorded. No deaths occurred in patients treated with up to ≤6 nT in the AntiHm group in all ages. A significant reduction in hospital admission was observed in the 2-7 nT groups either below or over 60 years old [Odds Ratio (OR) NoAntiHm/AntiHm = 1.76-1.32, respectively, in NoVAC or VAC (OR = 2.10 overall] and in the older ≥8 nT group (OR = 2.08 in NoVac]. In conclusion, patients with chronic antihistamine prescriptions, alone or with polypharmacy, showed reduced hospital admission and mortality rates, suggesting the safety of antihistamine treatment and the need to confirm its effectiveness in a prospective trial.
Supplementary Materials: The following supporting information can be downloaded at: https: //www.mdpi.com/article/10.3390/microorganisms12122589/s1 .
Conflicts of Interest: The authors declare no conflict of interest.
References
Aharandi, Pakdaman, Medghalchi, Kimia, Kazemian et al., A randomized open-label clinical trial on the effect of Amantadine on post COVID 19 fatigue, Sci. Rep, doi:10.1038/s41598-024-51904-z
Ariza, Cano, Segura, Adan, Bargalló et al., Neuropsychological impairment in post-COVID-19 condition individuals with and without cognitive complaents, Front. Aging Neurosci
Aryal, Mowbray, Miroshnychenko, Strum, Dash et al., Evaluating methods for risk prediction of COVID-19 mortality in nursing home residents before and after vaccine availability: A retrospective cohort study, BMC Med. Res. Methodol, doi:10.1186/s12874-024-02189-3
Atalla, Zhang, Shehadeh, Mylona, Tsikala-Vafea et al., Clinical Presentation, Course, and Risk Factors Associated with Mortality in a Severe Outbreak of COVID-19 in Rhode Island, USA, Pathogens, doi:10.3390/pathogens10010008
Benjamini, Hochberg, Multiple Hypotheses Testing with Weights, Scand. J. Stat, doi:10.1111/1467-9469.00072
Bielza, Sanz, Zambrana, Arias, Malmierca et al., Clinical Characteristics, Frailty, and Mortality of Residents with COVID-19 in Nursing Homes of a Region of Madrid, J. Am. Med. Dir. Assoc
Blanco, Bonilla, Fremont-Smith, Villar Gómez De Las Heras, Antihistamines as an early treatment for COVID-19, Heliyon
Blanco, Bonilla, Homma, Suzuki, Fremont-Smith et al., Antihistamines and azithromycin as a treatment for COVID-19 on primary health care-A retrospective observational study in elderly patients, Pulm. Pharmacol. Ther
Brennan, Nadella, Zhao, Dima, Jordan-Martin et al., Oral famotidine versus placebo in non-hospitalised patients with COVID-19: A randomised, double-blind, data-intense, phase 2 clinical trial, Gut, doi:10.1136/gutjnl-2022-326952
Cortés-Borra, Aranda-Abreu, Amantadine in the prevention of clinical symptoms caused by SARS-CoV-2, Pharmacol. Rep
Dean, Sullivan, Soe, Openepi, Open Source Epidemiologic Statistics for Public Health, Versión
Dinnes, Deeks, Berhane, Taylor, Adriano et al., Cochrane COVID-19 Diagnostic Test Accuracy Group 2. Rapid point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection, Cochrane Database Syst. Rev
Fadel, Morrison, Vahia, Smith, Chaudhry et al., Early Short-Course Corticosteroids in Hospitalized Patients with COVID-19, Clin. Infect. Dis, doi:10.1093/cid/ciaa601
Fischhuber, Bánki, Kimpel, Kragl, Rössler et al., Antiviral Potential of Azelastine against Major Respiratory Viruses, Viruses, doi:10.3390/v15122300
Fung, Baik, Baye, Zheng, Huser et al., Effect of common maintenance drugs on the risk and severity of COVID-19 in elderly patients, PLoS ONE, doi:10.1371/journal.pone.0266922
Ghasemi, Darvishi, Salari, Hosseinian-Far, Akbari et al., Global prevalence of polypharmacy among the COVID-19 patients: A comprehensive systematic review and meta-analysis of observational studies, Trop. Med. Health, doi:10.1186/s41182-022-00456-x
Glueck, Mandel, Karimpour-Fard, Hunter, Muller, Exact Calculations of Average Power for the Benjamini-Hochberg Procedure, Int. J. Biostat, doi:10.2202/1557-4679.1103
Gordon, Jang, Bouhaddou, Xu, Obernier et al., A SARS-CoV-2 Protein Interaction Map Reveals Targets for Drug-Repurposing, Nature, doi:10.1038/s41586-020-2286-9
Heymann, The Elderly and the COVID-19 19 Crisis: A Chronicle of Deaths Foretold, in Isolation and Total Indifference, Front. Public Health, doi:10.3389/fpubh.2020.602982
Juanes-González, Calderón-Valdiviezo, Losa-Puig, Valls-Foix, González-Salvador et al., COVID-19 First and Delta Waves in Relation to ACEI, ARB, Influenza Vaccination, and Comorbidity in a North Metropolitan Barcelona Health Consortium, doi:10.1101/2021.11.17.21265440
Klussmann, Grosheva, Meiser, Lehmann, Nagy et al., Early intervention with azelastine nasal spray may reduce viral load in SARS-CoV-2 infected patients, Sci. Rep
Kucirka, Lauer, Laeyendecker, Boon, Lessler, Variation in False-Negative Rate of Reverse Transcriptase Polymerase Chain Reaction-Based SARS-CoV-2 Tests by Time Since Exposure, Ann. Intern. Med
Mashauri, COVID-19 histamine theory: Why antihistamines should be incorporated as the basic component in COVID-19 management?, Health Sci. Rep, doi:10.1002/hsr2.1109
Meng, Xiao, Zhang, He, Ou et al., Renin-angiotensin System inhibitors improve the clinic outcomes of COVID-19 patients with hypertension, Emerg. Microbes Infect
Menzel, Akbarshahi, Bjermer, Uller, Azithromycin induces anti-viral effects in cultured bronchial epithelial cells from COPD patients, Sci. Rep, doi:10.1038/srep28698
Momtazmanesh, Ansari, Izadi, Shobeiri, Vatankhah et al., Effect of famotidine on cognitive and behavioral dysfunctions induced in post-COVID-19 infection: A randomized, double-blind, and placebo-controlled study, J. Psychosom. Res, doi:10.1016/j.jpsychores.2023.111389
Oh, Adnan, Cho, Network Pharmacology Study to Elucidate the Key Targets of Underlying Antihistamines against COVID-19, Curr. Issues Mol. Biol, doi:10.3390/cimb44040109
Owen, Conroy, Taub, Jones, Bryden et al., Comparing associations between frailty and mortality in hospitalised older adults with or without COVID-19 infection: A retrospective observational study using electronic health records, Age Ageing, doi:10.1093/ageing/afaa167
Puigdellívol-Sánchez, Juanes-González, Calderón-Valdiviezo, Valls-Foix, González-Salvador et al., COVID-19 in Relation to Polypharmacy and Immunization (2020-2024), Viruses, doi:10.3390/v16101533
Ragni, Marino, Formisano, Bisaccia, Scaltriti et al., Association between exposure to influenza vaccination and COVID-19 diagnosis and outcomes, Vaccines, doi:10.3390/vaccines8040675
Remuzzi, Remuzzi, COVID-19 and Italy: What next?, Lancet
Reznikov, Norris, Vashisht, Bluhm, Li et al., Identification of antiviral antihistamines for COVID-19 repurposing, Biochem. Biophys. Res. Commun
Saeedi-Boroujeni, Nashibi, Ghadiri, Nakajima, Salmanzadeh et al., Tranilast as an Adjunctive Therapy in Hospitalized Patients with Severe COVID-19: A Randomized Controlled Trial, Arch. Med. Res, doi:10.1016/j.arcmed.2022.03.002
Savarese, Benson, Sundström, Lund, Association between renin-angiotensin-aldosterone system inhibitor use and COVID-19 hospitalization and death: A 1.4 million patient nationwide registry analysis, Eur. J. Hearth Fail, doi:10.1002/ejhf.2060
Taghioff, Slavin, Holton, Singh, Examining the potential benefits of the influenza vaccine against SARS-CoV-2: A retrospective cohort analysis of 75754 patients, PLoS ONE, doi:10.1371/journal.pone.0255541
Tortorici, Walls, Lang, Wang, Li et al., Structural basis for human coronavirus attachment to sialic acid receptors, Nat. Struct. Mol. Biol, doi:10.1038/s41594-019-0233-y
Tran, Sugamata, Hirose, Suzuki, Noguchi et al., Azithromycin, a 15-membered macrolide antibiotic, inhibits influenza A(H1N1)pdm09 virus infection by interfering with virus internalization process, J. Antibiot, doi:10.1038/s41429-019-0204-x
Verdechia, Angeli, Reboldi, Angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers and coronavirus, J. Hypertens, doi:10.1097/HJH.0000000000002469
Walzer, Estève, Barben, Menu, Cuenot et al., Impact of Influenza Vaccination on Mortality in the Oldest Old: A Propensity Score-Matched Cohort Study, Vaccines, doi:10.3390/vaccines8030356
Yu, Liu, Ou, Li, Liu et al., The histamine receptor H1 acts as an alternative receptor for SARS-CoV-2, mBio, doi:10.1128/mbio.01088-24
Zeng, Langereis, Van Vliet, Huizinga, De Groot, Structure of coronavirus hemagglutinin-esterase offers insight into corona and influenza virus evolution, Proc. Natl. Acad. Sci
Zhong, Li, Wang, Identification of HRH1 as an alternative receptor for SARS-CoV-2: Insights from viral inhibition by repurposable antihistamines, mBio, doi:10.1128/mbio.01697-24
Zubiete-Franco, Tonks, Famotidine increases cellular phospho-tyrosine levels, Biochem. Biophys. Res. Commun, doi:10.1016/j.bbrc.2024.150763
DOI record:
{
"DOI": "10.3390/microorganisms12122589",
"ISSN": [
"2076-2607"
],
"URL": "http://dx.doi.org/10.3390/microorganisms12122589",
"abstract": "<jats:p>No hospitalizations or deaths occurred in residents with the COVID-19 infection, treated with antihistamines and azithromycin, of two external nursing homes during the first wave. We assessed whether patients receiving chronic antihistamines in our institution showed better clinical evolution. COVID-19 admissions and related deaths in the public Hospital of Terrassa (n = 1461) during the pandemic period (11 March 2020–5 May 2023) and cases (n = 32,888) during the period of full suspicion diagnosis (1 June 2020–23 March 2022) were referred to as the number of chronic treatments (nT) including or not including antihistamines (AntiHm or NOAntiHm), and their vaccination status before the first infection (VAC or NoVAC) in our assigned population (n = 140,681 at March 2020) was recorded. No deaths occurred in patients treated with up to ≤6 nT in the AntiHm group in all ages. A significant reduction in hospital admission was observed in the 2–7 nT groups either below or over 60 years old [Odds Ratio (OR) NoAntiHm/AntiHm = 1.76–1.32, respectively, in NoVAC or VAC (OR = 2.10 overall] and in the older ≥8 nT group (OR = 2.08 in NoVac]. In conclusion, patients with chronic antihistamine prescriptions, alone or with polypharmacy, showed reduced hospital admission and mortality rates, suggesting the safety of antihistamine treatment and the need to confirm its effectiveness in a prospective trial.</jats:p>",
"alternative-id": [
"microorganisms12122589"
],
"author": [
{
"ORCID": "https://orcid.org/0000-0001-7239-2953",
"affiliation": [
{
"name": "Medicina de Familia, CAP Anton de Borja-Centre Universitari, c/Marconi-Cantonada Edison s/n, Consorci Sanitari de Terrassa (CST), 08191 Rubí, Spain"
},
{
"name": "Human Anatomy and Embryology Unit, Faculty of Medicine, c/Casanova 143, Universitat de Barcelona, 08036 Barcelona, Spain"
}
],
"authenticated-orcid": false,
"family": "Puigdellívol-Sánchez",
"given": "Anna",
"sequence": "first"
},
{
"ORCID": "https://orcid.org/0000-0002-8682-6000",
"affiliation": [
{
"name": "Medicina de Familia, CAP Anton de Borja-Centre Universitari, c/Marconi-Cantonada Edison s/n, Consorci Sanitari de Terrassa (CST), 08191 Rubí, Spain"
}
],
"authenticated-orcid": false,
"family": "Juanes-González",
"given": "Marta",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Medicina de Familia, CAP Anton de Borja-Centre Universitari, c/Marconi-Cantonada Edison s/n, Consorci Sanitari de Terrassa (CST), 08191 Rubí, Spain"
}
],
"family": "Calderón-Valdiviezo",
"given": "Ana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Medicina de Familia, CAP Anton de Borja-Centre Universitari, c/Marconi-Cantonada Edison s/n, Consorci Sanitari de Terrassa (CST), 08191 Rubí, Spain"
},
{
"name": "Hospital Álvaro Cunqueiro, Estrada de Clara Campoamor 341, 36213 Vigo, Spain"
}
],
"family": "Losa-Puig",
"given": "Helena",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-1016-4039",
"affiliation": [
{
"name": "Medicina de Familia, CAP Anton de Borja-Centre Universitari, c/Marconi-Cantonada Edison s/n, Consorci Sanitari de Terrassa (CST), 08191 Rubí, Spain"
}
],
"authenticated-orcid": false,
"family": "Valls-Foix",
"given": "Roger",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0009-0009-9012-2899",
"affiliation": [
{
"name": "Management, Control and Information Analysis Unit, Hospital de Terrassa, Consorci Sanitari de Terrassa (CST), Carretera de Torrebonica s/n, 08227 Terrassa, Spain"
}
],
"authenticated-orcid": false,
"family": "González-Salvador",
"given": "Marta",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-2301-2509",
"affiliation": [
{
"name": "Medicina de Familia, CAP Anton de Borja-Centre Universitari, c/Marconi-Cantonada Edison s/n, Consorci Sanitari de Terrassa (CST), 08191 Rubí, Spain"
}
],
"authenticated-orcid": false,
"family": "Lozano-Paz",
"given": "Celia",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-3527-4242",
"affiliation": [
{
"name": "Intelligence for Primary Care Research Group, Foundation University Institute for Primary Health Care Research Jordi Gol i Gurina, 08242 Manresa, Spain"
},
{
"name": "Unitat de Recerca i Innovació, Gerència d‘Atenció Primària i a la Comunitat de la Catalunya Central, Institut Català de la Salut, 08242 Manresa, Spain"
}
],
"authenticated-orcid": false,
"family": "Vidal-Alaball",
"given": "Josep",
"sequence": "additional"
}
],
"container-title": "Microorganisms",
"container-title-short": "Microorganisms",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
12,
16
]
],
"date-time": "2024-12-16T11:48:39Z",
"timestamp": 1734349719000
},
"deposited": {
"date-parts": [
[
2024,
12,
16
]
],
"date-time": "2024-12-16T12:53:55Z",
"timestamp": 1734353635000
},
"funder": [
{
"DOI": "10.13039/501100002809",
"award": [
"SLT021/21/000002"
],
"doi-asserted-by": "crossref",
"id": [
{
"asserted-by": "crossref",
"id": "10.13039/501100002809",
"id-type": "DOI"
}
],
"name": "Generalitat de Catalunya"
}
],
"indexed": {
"date-parts": [
[
2024,
12,
17
]
],
"date-time": "2024-12-17T05:11:32Z",
"timestamp": 1734412292276,
"version": "3.30.2"
},
"is-referenced-by-count": 0,
"issue": "12",
"issued": {
"date-parts": [
[
2024,
12,
13
]
]
},
"journal-issue": {
"issue": "12",
"published-online": {
"date-parts": [
[
2024,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
12,
13
]
],
"date-time": "2024-12-13T00:00:00Z",
"timestamp": 1734048000000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2076-2607/12/12/2589/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "2589",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2024,
12,
13
]
]
},
"published-online": {
"date-parts": [
[
2024,
12,
13
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"key": "ref_1",
"unstructured": "Ministerio de Ciencia, Innovación y Universidades (2024, June 30). Instituto de Salud Carlos III. Available online: https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/MoMo/Documents/informesMoMo2020/MoMo_Situacion%20a%2031%20de%20marzo_CNE.pdf."
},
{
"DOI": "10.1016/S0140-6736(20)30627-9",
"article-title": "COVID-19 and Italy: What next?",
"author": "Remuzzi",
"doi-asserted-by": "crossref",
"first-page": "1225",
"journal-title": "Lancet",
"key": "ref_2",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/j.jamda.2020.12.003",
"article-title": "Clinical Characteristics, Frailty, and Mortality of Residents with COVID-19 in Nursing Homes of a Region of Madrid",
"author": "Bielza",
"doi-asserted-by": "crossref",
"first-page": "245",
"journal-title": "J. Am. Med. Dir. Assoc.",
"key": "ref_3",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.3389/fpubh.2020.602982",
"doi-asserted-by": "crossref",
"key": "ref_4",
"unstructured": "Heymann, D. (2020). The Elderly and the COVID-19 19 Crisis: A Chronicle of Deaths Foretold, in Isolation and Total Indifference. Front. Public Health, 8."
},
{
"DOI": "10.3390/pathogens10010008",
"doi-asserted-by": "crossref",
"key": "ref_5",
"unstructured": "Atalla, E., Zhang, R., Shehadeh, F., Mylona, E.K., Tsikala-Vafea, M., Kalagara, S., Henseler, L., Chan, P.A., and Mylonakis, E. (2020). Clinical Presentation, Course, and Risk Factors Associated with Mortality in a Severe Outbreak of COVID-19 in Rhode Island, USA, April-June 2020. Pathogens, 10."
},
{
"DOI": "10.1016/j.pupt.2021.101989",
"article-title": "Antihistamines and azithromycin as a treatment for COVID-19 on primary health care—A retrospective observational study in elderly patients",
"author": "Homma",
"doi-asserted-by": "crossref",
"first-page": "101989",
"journal-title": "Pulm. Pharmacol. Ther.",
"key": "ref_6",
"volume": "67",
"year": "2021"
},
{
"DOI": "10.1016/j.heliyon.2023.e15772",
"article-title": "Antihistamines as an early treatment for COVID-19",
"doi-asserted-by": "crossref",
"first-page": "e15772",
"journal-title": "Heliyon",
"key": "ref_7",
"volume": "9",
"year": "2023"
},
{
"DOI": "10.1073/pnas.0800502105",
"article-title": "Structure of coronavirus hemagglutinin-esterase offers insight into corona and influenza virus evolution",
"author": "Zeng",
"doi-asserted-by": "crossref",
"first-page": "9065",
"journal-title": "Proc. Natl. Acad. Sci. USA",
"key": "ref_8",
"volume": "105",
"year": "2008"
},
{
"DOI": "10.1038/s41594-019-0233-y",
"article-title": "Structural basis for human coronavirus attachment to sialic acid receptors",
"author": "Tortorici",
"doi-asserted-by": "crossref",
"first-page": "481",
"journal-title": "Nat. Struct. Mol. Biol.",
"key": "ref_9",
"volume": "26",
"year": "2019"
},
{
"DOI": "10.1080/22221751.2020.1746200",
"article-title": "Renin-angiotensin System inhibitors improve the clinic outcomes of COVID-19 patients with hypertension",
"author": "Meng",
"doi-asserted-by": "crossref",
"first-page": "757",
"journal-title": "Emerg. Microbes Infect.",
"key": "ref_10",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1097/HJH.0000000000002469",
"article-title": "Angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers and coronavirus",
"author": "Verdechia",
"doi-asserted-by": "crossref",
"first-page": "1190",
"journal-title": "J. Hypertens.",
"key": "ref_11",
"volume": "38",
"year": "2020"
},
{
"DOI": "10.1002/ejhf.2060",
"article-title": "Association between renin-angiotensin-aldosterone system inhibitor use and COVID-19 hospitalization and death: A 1.4 million patient nationwide registry analysis",
"author": "Savarese",
"doi-asserted-by": "crossref",
"first-page": "476",
"journal-title": "Eur. J. Hearth Fail.",
"key": "ref_12",
"volume": "23",
"year": "2021"
},
{
"DOI": "10.3390/vaccines8030356",
"doi-asserted-by": "crossref",
"key": "ref_13",
"unstructured": "Walzer, P., Estève, C., Barben, J., Menu, D., Cuenot, C., Manckoundia, P., and Putot, A. (2020). Impact of Influenza Vaccination on Mortality in the Oldest Old: A Propensity Score-Matched Cohort Study. Vaccines, 8."
},
{
"DOI": "10.3390/vaccines8040675",
"doi-asserted-by": "crossref",
"key": "ref_14",
"unstructured": "Ragni, P., Marino, M., Formisano, D., Bisaccia, E., Scaltriti, S., Bedeschi, E., and Grilli, R. (2020). Association between exposure to influenza vaccination and COVID-19 diagnosis and outcomes. Vaccines, 8."
},
{
"DOI": "10.1371/journal.pone.0255541",
"doi-asserted-by": "crossref",
"key": "ref_15",
"unstructured": "Taghioff, S.M., Slavin, B.R., Holton, T., and Singh, D. (2021). Examining the potential benefits of the influenza vaccine against SARS-CoV-2: A retrospective cohort analysis of 75754 patients. PLoS ONE, 16."
},
{
"DOI": "10.1101/2021.11.17.21265440",
"doi-asserted-by": "crossref",
"key": "ref_16",
"unstructured": "Juanes-González, M., Calderón-Valdiviezo, A., Losa-Puig, H., Valls-Foix, R., González-Salvador, M., León-Pérez, M., Pueyo-Antón, L., Lozano-Paz, C., Franco-Romero, M., and Vidal-Alaball, J. (2021). COVID-19 First and Delta Waves in Relation to ACEI, ARB, Influenza Vaccination, and Comorbidity in a North Metropolitan Barcelona Health Consortium. medRxiv."
},
{
"DOI": "10.3390/v16101533",
"doi-asserted-by": "crossref",
"key": "ref_17",
"unstructured": "Puigdellívol-Sánchez, A., Juanes-González, M., Calderón-Valdiviezo, A., Valls-Foix, R., González-Salvador, M., Lozano-Paz, C., and Vidal-Alaball, J. (2024). COVID-19 in Relation to Polypharmacy and Immunization (2020–2024). Viruses, 16."
},
{
"DOI": "10.1093/ageing/afaa167",
"article-title": "Comparing associations between frailty and mortality in hospitalised older adults with or without COVID-19 infection: A retrospective observational study using electronic health records",
"author": "Owen",
"doi-asserted-by": "crossref",
"first-page": "307",
"journal-title": "Age Ageing",
"key": "ref_18",
"volume": "50",
"year": "2020"
},
{
"DOI": "10.1186/s41182-022-00456-x",
"article-title": "Global prevalence of polypharmacy among the COVID-19 patients: A comprehensive systematic review and meta-analysis of observational studies",
"author": "Ghasemi",
"doi-asserted-by": "crossref",
"first-page": "60",
"journal-title": "Trop. Med. Health",
"key": "ref_19",
"volume": "50",
"year": "2022"
},
{
"DOI": "10.1186/s12874-024-02189-3",
"doi-asserted-by": "crossref",
"key": "ref_20",
"unstructured": "Aryal, K., Mowbray, F.I., Miroshnychenko, A., Strum, R.P., Dash, D., Hillmer, M.P., Malikov, K., Costa, A.P., and Jones, A. (2024). Evaluating methods for risk prediction of COVID-19 mortality in nursing home residents before and after vaccine availability: A retrospective cohort study. BMC Med. Res. Methodol., 24."
},
{
"key": "ref_21",
"unstructured": "(2024, December 05). Available online: https://catsalut.gencat.cat/web/.content/minisite/catsalut/proveidors_professionals/registres_catalegs/documents/informe-poblacio-referencia-2024.pdf."
},
{
"key": "ref_22",
"unstructured": "(2024, December 05). Available online: https://observatorisalut.gencat.cat/ca/observatori-desigualtats-salut/dades_obertes/index.html."
},
{
"key": "ref_23",
"unstructured": "Dean, A.G., Sullivan, K.M., and Soe, M.M. (2024, November 15). OpenEpi: Open Source Epidemiologic Statistics for Public Health, Versión. Available online: https://www.openepi.com/Menu/OE_Menu.htm."
},
{
"DOI": "10.1111/1467-9469.00072",
"article-title": "Multiple Hypotheses Testing with Weights",
"author": "Benjamini",
"doi-asserted-by": "crossref",
"first-page": "407",
"journal-title": "Scand. J. Stat.",
"key": "ref_24",
"volume": "24",
"year": "1997"
},
{
"DOI": "10.2202/1557-4679.1103",
"doi-asserted-by": "crossref",
"key": "ref_25",
"unstructured": "Glueck, D.H., Mandel, J., Karimpour-Fard, A., Hunter, L., and Muller, K.E. (2008). Exact Calculations of Average Power for the Benjamini-Hochberg Procedure. Int. J. Biostat., 4."
},
{
"DOI": "10.1093/cid/ciaa601",
"article-title": "Early Short-Course Corticosteroids in Hospitalized Patients with COVID-19",
"author": "Fadel",
"doi-asserted-by": "crossref",
"first-page": "2114",
"journal-title": "Clin. Infect. Dis.",
"key": "ref_26",
"volume": "71",
"year": "2020"
},
{
"DOI": "10.1038/srep28698",
"article-title": "Azithromycin induces anti-viral effects in cultured bronchial epithelial cells from COPD patients",
"author": "Menzel",
"doi-asserted-by": "crossref",
"first-page": "28698",
"journal-title": "Sci. Rep.",
"key": "ref_27",
"volume": "6",
"year": "2016"
},
{
"DOI": "10.1038/s41429-019-0204-x",
"article-title": "Azithromycin, a 15-membered macrolide antibiotic, inhibits influenza A(H1N1)pdm09 virus infection by interfering with virus internalization process",
"author": "Tran",
"doi-asserted-by": "crossref",
"first-page": "759",
"journal-title": "J. Antibiot.",
"key": "ref_28",
"volume": "72",
"year": "2019"
},
{
"DOI": "10.3390/cimb44040109",
"article-title": "Network Pharmacology Study to Elucidate the Key Targets of Underlying Antihistamines against COVID-19",
"author": "Oh",
"doi-asserted-by": "crossref",
"first-page": "1597",
"journal-title": "Curr. Issues Mol. Biol.",
"key": "ref_29",
"volume": "44",
"year": "2022"
},
{
"DOI": "10.1016/j.arcmed.2022.03.002",
"article-title": "Tranilast as an Adjunctive Therapy in Hospitalized Patients with Severe COVID-19: A Randomized Controlled Trial",
"author": "Nashibi",
"doi-asserted-by": "crossref",
"first-page": "368",
"journal-title": "Arch. Med. Res.",
"key": "ref_30",
"volume": "53",
"year": "2022"
},
{
"DOI": "10.1038/s41598-023-32546-z",
"doi-asserted-by": "crossref",
"key": "ref_31",
"unstructured": "Klussmann, J.P., Grosheva, M., Meiser, P., Lehmann, C., Nagy, E., Szijártó, V., Nagy, G., Konrat, R., Flegel, M., and Holzer, F. (2023). Early intervention with azelastine nasal spray may reduce viral load in SARS-CoV-2 infected patients. Sci. Rep., 13."
},
{
"DOI": "10.1136/gutjnl-2022-326952",
"article-title": "Oral famotidine versus placebo in non-hospitalised patients with COVID-19: A randomised, double-blind, data-intense, phase 2 clinical trial",
"author": "Brennan",
"doi-asserted-by": "crossref",
"first-page": "879",
"journal-title": "Gut",
"key": "ref_32",
"volume": "71",
"year": "2022"
},
{
"DOI": "10.1016/j.jpsychores.2023.111389",
"article-title": "Effect of famotidine on cognitive and behavioral dysfunctions induced in post-COVID-19 infection: A randomized, double-blind, and placebo-controlled study",
"author": "Momtazmanesh",
"doi-asserted-by": "crossref",
"first-page": "111389",
"journal-title": "J. Psychosom. Res.",
"key": "ref_33",
"volume": "172",
"year": "2023"
},
{
"DOI": "10.1016/j.bbrc.2024.150763",
"doi-asserted-by": "crossref",
"key": "ref_34",
"unstructured": "Zubiete-Franco, I., and Tonks, N.K. (2024). Famotidine increases cellular phospho-tyrosine levels. Biochem. Biophys. Res. Commun., 734."
},
{
"DOI": "10.1371/journal.pone.0266922",
"doi-asserted-by": "crossref",
"key": "ref_35",
"unstructured": "Fung, K.W., Baik, S.H., Baye, F., Zheng, Z., Huser, V., and McDonald, C. (2022). Effect of common maintenance drugs on the risk and severity of COVID-19 in elderly patients. PLoS ONE, 17."
},
{
"DOI": "10.1016/j.bbrc.2020.11.095",
"article-title": "Identification of antiviral antihistamines for COVID-19 repurposing",
"author": "Reznikov",
"doi-asserted-by": "crossref",
"first-page": "173",
"journal-title": "Biochem. Biophys. Res. Commun.",
"key": "ref_36",
"volume": "538",
"year": "2021"
},
{
"DOI": "10.3390/v15122300",
"doi-asserted-by": "crossref",
"key": "ref_37",
"unstructured": "Fischhuber, K., Bánki, Z., Kimpel, J., Kragl, N., Rössler, A., Bolze, A., Muellauer, B., Angerer, J., Nagy, G., and Nagy, E. (2023). Antiviral Potential of Azelastine against Major Respiratory Viruses. Viruses, 15."
},
{
"DOI": "10.1002/hsr2.1109",
"article-title": "COVID-19 histamine theory: Why antihistamines should be incorporated as the basic component in COVID-19 management?",
"author": "Mashauri",
"doi-asserted-by": "crossref",
"first-page": "e1109",
"journal-title": "Health Sci. Rep.",
"key": "ref_38",
"volume": "6",
"year": "2023"
},
{
"DOI": "10.1038/s41586-020-2286-9",
"article-title": "A SARS-CoV-2 Protein Interaction Map Reveals Targets for Drug-Repurposing",
"author": "Gordon",
"doi-asserted-by": "crossref",
"first-page": "459",
"journal-title": "Nature",
"key": "ref_39",
"volume": "583",
"year": "2020"
},
{
"DOI": "10.1128/mbio.01088-24",
"article-title": "The histamine receptor H1 acts as an alternative receptor for SARS-CoV-2",
"author": "Yu",
"doi-asserted-by": "crossref",
"first-page": "e01088-24",
"journal-title": "mBio",
"key": "ref_40",
"volume": "15",
"year": "2024"
},
{
"DOI": "10.1128/mbio.01697-24",
"article-title": "Identification of HRH1 as an alternative receptor for SARS-CoV-2: Insights from viral inhibition by repurposable antihistamines",
"author": "Zhong",
"doi-asserted-by": "crossref",
"first-page": "e01697-24",
"journal-title": "mBio",
"key": "ref_41",
"volume": "15",
"year": "2024"
},
{
"DOI": "10.7326/M20-1495",
"article-title": "Variation in False-Negative Rate of Reverse Transcriptase Polymerase Chain Reaction-Based SARS-CoV-2 Tests by Time Since Exposure",
"author": "Kucirka",
"doi-asserted-by": "crossref",
"first-page": "M20",
"journal-title": "Ann. Intern. Med.",
"key": "ref_42",
"volume": "173",
"year": "2020"
},
{
"key": "ref_43",
"unstructured": "(2024, September 18). Available online: https://iris.who.int/bitstream/handle/10665/360580/WHO-2019-nCoV-SurveillanceGuidance-2022.2-eng.pdf."
},
{
"article-title": "Cochrane COVID-19 Diagnostic Test Accuracy Group 2. Rapid point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection",
"author": "Dinnes",
"first-page": "CD013705",
"journal-title": "Cochrane Database Syst. Rev.",
"key": "ref_44",
"volume": "3",
"year": "2021"
},
{
"DOI": "10.1007/s43440-021-00231-5",
"article-title": "Aranda-Abreu GE. Amantadine in the prevention of clinical symptoms caused by SARS-CoV-2",
"doi-asserted-by": "crossref",
"first-page": "962",
"journal-title": "Pharmacol. Rep.",
"key": "ref_45",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.1038/s41598-024-51904-z",
"doi-asserted-by": "crossref",
"key": "ref_46",
"unstructured": "AHarandi, A.A., Pakdaman, H., Medghalchi, A., Kimia, N., Kazemian, A., Siavoshi, F., Barough, S.S., Esfandani, A., Hosseini, M.H., and Sobhanian, S.A. (2024). A randomized open-label clinical trial on the effect of Amantadine on post COVID 19 fatigue. Sci. Rep., 14."
},
{
"key": "ref_47",
"unstructured": "Generalitat de Catalunya (2024, July 04). Sistema d’Informació per a la Vigilància d’Infeccions a Catalunya. Available online: https://sivic.salut.gencat.cat/."
},
{
"key": "ref_48",
"unstructured": "University of Oxford-Oxford Martin School (2024, July 04). Official Data Collated by Our World in Data—Last Updated 3 July 2024—Processed by Our World in Data. Available online: https://ourworldindata.org/grapher/current-covid-hospitalizations-per-million."
},
{
"DOI": "10.3389/fnagi.2022.1029842",
"doi-asserted-by": "crossref",
"key": "ref_49",
"unstructured": "Ariza, M., Cano, N., Segura, B., Adan, A., Bargalló, N., Caldú, X., Campabadal, A., Jurado, M.A., Mataró, M., and Pueyo, R. (2022). Neuropsychological impairment in post-COVID-19 condition individuals with and without cognitive complaents. Front. Aging Neurosci., 14."
}
],
"reference-count": 49,
"references-count": 49,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2076-2607/12/12/2589"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "COVID-19 in Relation to Chronic Antihistamine Prescription",
"type": "journal-article",
"volume": "12"
}
