Efficacy and safety of colchicine treatment in patients with COVID-19: A prospective, multicenter, randomized clinical trial
et al., Phytotherapy Research, doi:10.1002/ptr.7319, Feb 2022
Colchicine for COVID-19
5th treatment shown to reduce risk in
September 2020, now with p = 0.0000049 from 54 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
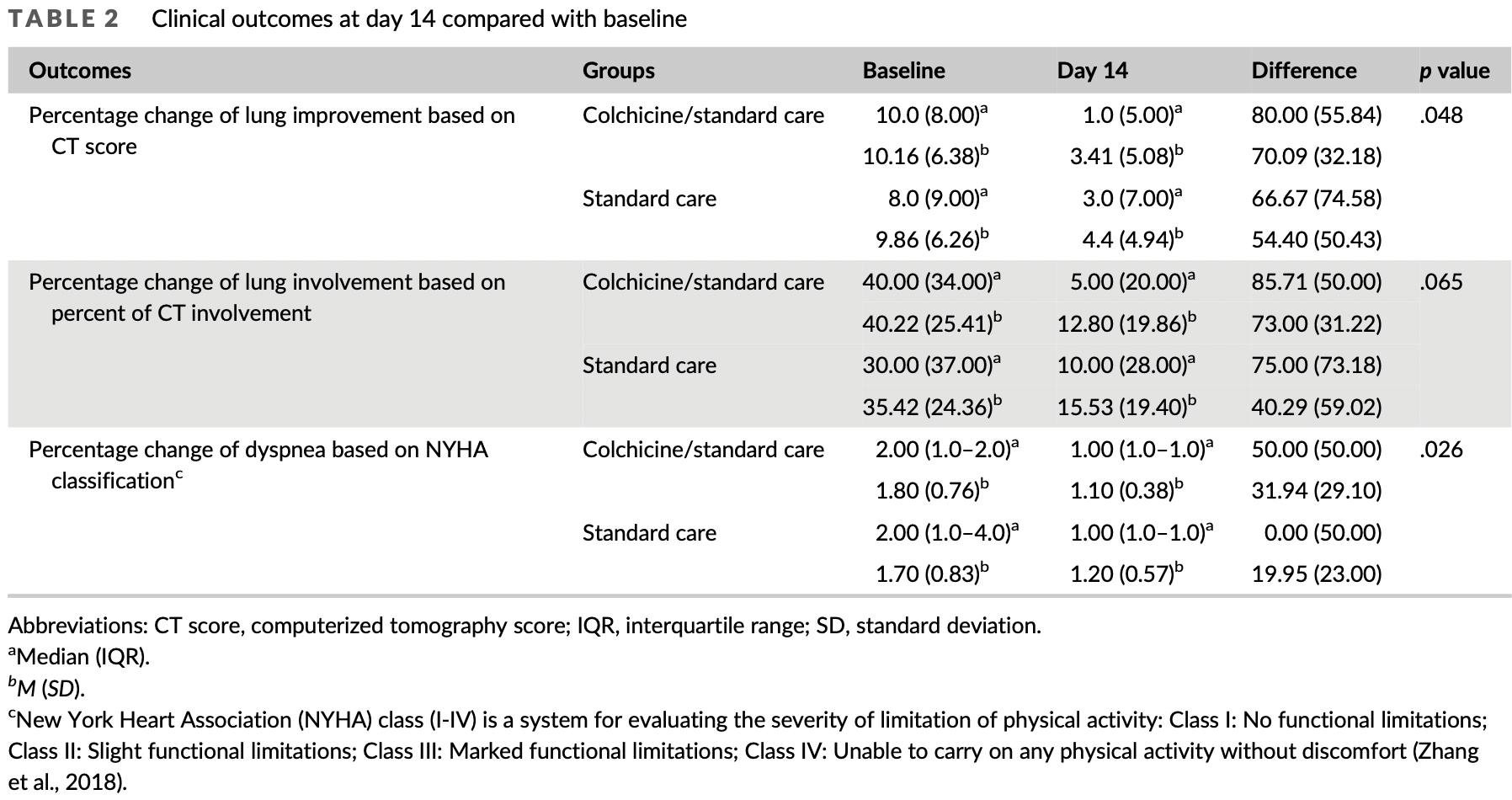
RCT 202 patients in Iran, 102 treated with colchicine, showing lower hospitalization and improved clinical outcomes with treatment.
|
risk of hospitalization, 72.8% lower, RR 0.27, p = 0.004, treatment 5 of 102 (4.9%), control 18 of 100 (18.0%), NNT 7.6.
|
|
relative improvement in dyspnea, 37.5% better, RR 0.62, p = 0.03, treatment 89, control 63, excluding 5 treatment and 37 control patients that needed hospitalization/other interventions.
|
|
relative improvement in Ct score, 22.4% better, RR 0.78, p = 0.048, treatment 89, control 63, excluding 5 treatment and 37 control patients that needed hospitalization/other interventions.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Pourdowlat et al., 2 Feb 2022, Randomized Controlled Trial, Iran, peer-reviewed, 18 authors, study period 26 March, 2020 - 30 September, 2020.
Efficacy and safety of colchicine treatment in patients with COVID ‐19: A prospective, multicenter, randomized clinical trial
Phytotherapy Research, doi:10.1002/ptr.7319
Colchicine has shown clinical benefits in the management of COVID-19 via its antiinflammatory effect. However, the exact role of colchicine in COVID-19 patients is unknown. The current clinical trial was performed on 202 patients with moderate to severe COVID-19. Patients were randomly assigned in a 1:1 ratio to receive up to a 3-day course of 0.5 mg colchicine followed by a 12-day course of 1 mg colchicine in combination with standard care or a 15-day course of standard care. Among 202 randomized patients, 153 completed the study and received colchicine/standard care or
CONFLICT OF INTEREST The authors declare that they have no conflicts of interest regarding the publication of this paper.
AUTHOR CONTRIBUTIONS
Guitti
References
Arcanjo, Logullo, Menezes, Giangiarulo, Dos Reis et al., The emerging role of neutrophil extracellular traps in severe acute respiratory syndrome coronavirus 2 (COVID-19), Scientific Reports
Beheshtian, Izadi, Kriegshauser, Kahrizi, Mehr et al., Prevalence of common MEFV mutations and carrier frequencies in a large cohort of Iranian populations, Journal of Genetics
Davatchi, Shahram, Chams-Davatchi, Shams, Nadji et al., Behcet's disease in Iran: Analysis of 6500 cases
Deftereos, Giannopoulos, Vrachatis, Siasos, Giotaki et al., Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: The GRECCO-19 randomized clinical trial, JAMA Network Open
Demidowich, Davis, Dedhia, Yanovski, Colchicine to decrease NLRP3-activated inflammation and improve obesityrelated metabolic dysregulation, Medical Hypotheses
Dupuis, Sirois, Rhéaume, Nguyen, Clavet-Lanthier et al., Colchicine reduces lung injury in experimental acute respiratory distress syndrome, PLoS One
Fiorucci, Lucantoni, Paone, Zotti, Li Bianchi et al., Colchicine, cyclophosphamide and prednisone in the treatment of mild-moderate idiopathic pulmonary fibrosis: Comparison of three currently available therapeutic regimens, European Review for Medical and Pharmacological Sciences
Francone, Iafrate, Masci, Coco, Cilia et al., Chest CT score in COVID-19 patients: Correlation with disease severity and short-term prognosis, European Radiology
Freeman, Swartz, Targeting the NLRP3 inflammasome in severe COVID-19, Frontiers in Immunology
George, Wells, Jenkins, Pulmonary fibrosis and COVID-19: The potential role for antifibrotic therapy, The Lancet Respiratory Medicine
Jain, Effect of COVID-19 on the organs, Cureus
Jimeno, Ventura, Castellano, García-Adasme, Miranda et al., Prognostic implications of neutrophil-lymphocyte ratio in COVID-19, European Journal of Clinical Investigation
Kommoss, Schwab, Tavernar, Schreck, Wagner et al., The pathology of severe COVID-19-related lung damage: Mechanistic and therapeutic implications, Deutsches Ärzteblatt International
Liu, Lei, Liao, Shi, Li et al., Semiquantitative analysis for the dynamic chest CT imaging features from onset to recovery in severe and critical COVID-19, Radiology of Infectious Diseases
Mansouri, Marjani, Tabarsi, Garnier, Mansouri, Successful treatment of Covid-19 associated cytokine release syndrome with colchicine. A case report and review of literature, Immunological Investigations
Mo, Jian, Su, Chen, Peng et al., Abnormal pulmonary function in COVID-19 patients at time of hospital discharge, European Respiratory Journal
Nasiripour, Zamani, Farasatinasab, Can colchicine as an old anti-inflammatory agent be effective in COVID-19?, Journal of Clinical Pharmacology
Pan, Ye, Sun, Gui, Liang et al., Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia, Radiology
Reddel, Pennings, Curnow, Chen, Kritharides, Procoagulant effects of low-level platelet activation and its inhibition by colchicine, Thrombosis and Haemostasis
Robertson, Payet, Martinez, Barraclough, Celermajer et al., Colchicine acutely suppresses the NLRP3 Inflammasome in acute coronary syndrome patients monocytes, Circulation
Sahebnasagh, Saghafi, Safdari, Khataminia, Sadremomtaz et al., Neutrophil Elastase inhibitor (Sivelestat), may be a promising therapeutic option for Management of Acute Lung Injury/acute respiratory distress syndrome or disseminated intravascular coagulation in COVID-19, Journal of Clinical Pharmacy and Therapeutics
Sandhu, Tieng, Chilimuri, Franchin, A case control study to evaluate the impact of colchicine on patients admitted to the hospital with moderate to severe COVID-19 infection, Canadian Journal of Infectious Diseases and Medical Microbiology
Scarsi, Piantoni, Colombo, Air O, Richini et al., Association between treatment with colchicine and improved survival in a single-Centre cohort of adult hospitalised patients with COVID-19 pneumonia and acute respiratory distress syndrome, Annals of the Rheumatic Diseases
Seçkin, Bütün, Bas, Takcı, Kalkan, Effects of colchicine treatment on mean platelet volume and the inflammatory markers in recurrent aphthous stomatitis, Journal of Dermatological Treatment
Slobodnick, Shah, Krasnokutsky, Pillinger, Update on colchicine, Rheumatology
Song, Liu, Shi, Chu, Zhang et al., SARS-CoV-2 induced diarrhoea as onset symptom in patient with COVID-19, Gut
Spinner, Gottlieb, Criner, Opez, Cattelan et al., Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: A randomized clinical trial, JAMA
Tardif, Bouabdallaoui, L'allier, Gaudet, Shah et al., Efficacy of colchicine in nonhospitalized patients with COVID-19, Medrxiv, doi:10.1101/2021.01.26.21250494
Vaidya, Tucker, Kurup, Khandkar, Pandzic et al., Colchicine inhibits neutrophil extracellular trap formation in patients with acute coronary syndrome after percutaneous coronary intervention, Journal of the American Heart Association
Yurdakul, Mat, Tüzün, Özyazgan, Hamuryudan et al., A double-blind trial of colchicine in Behçet's syndrome, Arthritis and Rheumatism
Zhang, Ma, Shanahan, Munroe, Horn et al., Efficacy and safety of colchicine treatment in patients with COVID-19: A prospective, multicenter, randomized clinical trial, BMC Medical Informatics and Decision Making
DOI record:
{
"DOI": "10.1002/ptr.7319",
"ISSN": [
"0951-418X",
"1099-1573"
],
"URL": "http://dx.doi.org/10.1002/ptr.7319",
"alternative-id": [
"10.1002/ptr.7319"
],
"archive": [
"Portico"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2021-05-10"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2021-10-12"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2022-02-02"
}
],
"author": [
{
"affiliation": [
{
"name": "Chronic Respiratory Diseases Research Centre, National Research Institute of Tuberculosis and Lung Diseases (NRITLD), Shahid Beheshti University of Medical Sciences Tehran Iran"
}
],
"family": "Pourdowlat",
"given": "Guitti",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Clinical Pharmacy School of Pharmacy and Pharmaceutical Sciences Research Center, Shahid Sadoughi University of Medical Sciences Yazd Iran"
}
],
"family": "Saghafi",
"given": "Fatemeh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medical Sciences Qom Branch, Islamic Azad University Qom Iran"
}
],
"family": "Mozafari",
"given": "Abolfazl",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Research Center, Department of Internal Medicine, School of Medicine, North Khorasan University of Medical Sciences Bojnurd Iran"
}
],
"family": "Sahebnasagh",
"given": "Adeleh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Chronic Respiratory Diseases Research Centre, National Research Institute of Tuberculosis and Lung Diseases (NRITLD), Shahid Beheshti University of Medical Sciences Tehran Iran"
}
],
"family": "Abedini",
"given": "Atefeh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pharmaceutics School of Pharmacy, Shahid Sadoughi University of Medical Sciences and Health Services Yazd Iran"
}
],
"family": "Nabi Meybodi",
"given": "Mohsen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Cardiology Tehranpars Hospital Tehran Iran"
}
],
"family": "Salehi Nezamabadi",
"given": "Ali",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Diseases Research Center, Shahid Sadoughi Hospital, Shahid Sadoughi University of Medical Sciences Yazd Iran"
}
],
"family": "Mousavinasab",
"given": "Seyed Ruhollah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Tracheal Diseases Research Center, National Research Institute of Tuberculosis and Lung Diseases (NRITLD) Shahid Beheshti University of Medical Sciences Tehran Iran"
}
],
"family": "Kiani",
"given": "Arda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine Air pollution and Respiratory Diseases Research Center, Ahvaz Jundishapur University of Medical Sciences Ahvaz Iran"
}
],
"family": "Raji",
"given": "Hanieh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pharmaceutical Sciences Research Center, School of Pharmacy, Student Research Committee, Shahid Sadoughi University of Medical Sciences Yazd Iran"
}
],
"family": "Soltani",
"given": "Nadia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Radiology Imam Hossein Hospital, Shahid Beheshti University of Medical Sciences Tehran Iran"
}
],
"family": "Gholmzadeh Baeis",
"given": "Mehdi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "NRITLD, Shahid Beheshti University of Medical Sciences Tehran Iran"
}
],
"family": "Eidani",
"given": "Esmaeil",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Radiology, Faculty of Medicine Shahid Sadoughi University of Medical Sciences Yazd Iran"
}
],
"family": "Sadeghi Yakhdani",
"given": "Abdolrahim",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medical Sciences Qom Branch, Islamic Azad University Qom Iran"
}
],
"family": "Movaseghi",
"given": "Fatemeh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pharmacognosy Research Laboratories and Herbal Analysis Services UK, University of Greenwich Kent UK"
}
],
"family": "Habtemariam",
"given": "Solomon",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Disease Research Center, Shahid Sadoughi Hospital, Shahid Sadoughi University of Medical Sciences and Health Services Yazd Iran"
}
],
"family": "Akhoundi Meybodi",
"given": "Zohreh",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9026-8710",
"affiliation": [
{
"name": "Department of Internal Medicine, Faculty of Medicine Shahid Sadoughi University of Medical Sciences Yazd Iran"
}
],
"authenticated-orcid": false,
"family": "Gholinataj Jelodar",
"given": "Mohsen",
"sequence": "additional"
}
],
"container-title": [
"Phytotherapy Research"
],
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2022,
2,
2
]
],
"date-time": "2022-02-02T11:39:20Z",
"timestamp": 1643801960000
},
"deposited": {
"date-parts": [
[
2022,
2,
2
]
],
"date-time": "2022-02-02T11:39:54Z",
"timestamp": 1643801994000
},
"indexed": {
"date-parts": [
[
2022,
2,
2
]
],
"date-time": "2022-02-02T12:14:13Z",
"timestamp": 1643804053260
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "print",
"value": "0951-418X"
},
{
"type": "electronic",
"value": "1099-1573"
}
],
"issued": {
"date-parts": [
[
2022,
2,
2
]
]
},
"language": "en",
"license": [
{
"URL": "http://onlinelibrary.wiley.com/termsAndConditions#vor",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
2,
2
]
],
"date-time": "2022-02-02T00:00:00Z",
"timestamp": 1643760000000
}
},
{
"URL": "http://doi.wiley.com/10.1002/tdm_license_1.1",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
2,
2
]
],
"date-time": "2022-02-02T00:00:00Z",
"timestamp": 1643760000000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/ptr.7319",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1002/ptr.7319",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/ptr.7319",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"prefix": "10.1002",
"published": {
"date-parts": [
[
2022,
2,
2
]
]
},
"published-online": {
"date-parts": [
[
2022,
2,
2
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1038/s41598-020-76781-0",
"article-title": "The emerging role of neutrophil extracellular traps in severe acute respiratory syndrome coronavirus 2 (COVID‐19)",
"author": "Arcanjo A.",
"doi-asserted-by": "crossref",
"first-page": "1",
"issue": "1",
"journal-title": "Scientific Reports",
"key": "e_1_2_13_2_1",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1007/s12041-016-0682-6",
"article-title": "Prevalence of common MEFV mutations and carrier frequencies in a large cohort of Iranian populations",
"author": "Beheshtian M.",
"doi-asserted-by": "crossref",
"first-page": "667",
"issue": "3",
"journal-title": "Journal of Genetics",
"key": "e_1_2_13_3_1",
"volume": "95",
"year": "2016"
},
{
"key": "e_1_2_13_4_1",
"unstructured": "ClinicalTrials.gov identifer. (2020).https://www.clinicaltrials.gov//"
},
{
"DOI": "10.1111/j.1756-185X.2010.01549.x",
"article-title": "Behcet's disease in Iran: Analysis of 6500 cases",
"author": "Davatchi F.",
"doi-asserted-by": "crossref",
"first-page": "367",
"issue": "4",
"journal-title": "International Journal of Rheumatic Diseases",
"key": "e_1_2_13_5_1",
"volume": "13",
"year": "2010"
},
{
"DOI": "10.1001/jamanetworkopen.2020.13136",
"article-title": "Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: The GRECCO‐19 randomized clinical trial",
"author": "Deftereos S. G.",
"doi-asserted-by": "crossref",
"first-page": "e2013136",
"issue": "6",
"journal-title": "JAMA Network Open",
"key": "e_1_2_13_6_1",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.1016/j.mehy.2016.04.039",
"article-title": "Colchicine to decrease NLRP3‐activated inflammation and improve obesity‐related metabolic dysregulation",
"author": "Demidowich A. P.",
"doi-asserted-by": "crossref",
"first-page": "67",
"journal-title": "Medical Hypotheses",
"key": "e_1_2_13_7_1",
"volume": "92",
"year": "2016"
},
{
"DOI": "10.1371/journal.pone.0242318",
"article-title": "Colchicine reduces lung injury in experimental acute respiratory distress syndrome",
"author": "Dupuis J.",
"doi-asserted-by": "crossref",
"first-page": "e0242318",
"issue": "12",
"journal-title": "PLoS One",
"key": "e_1_2_13_8_1",
"volume": "15",
"year": "2020"
},
{
"article-title": "Colchicine, cyclophosphamide and prednisone in the treatment of mild‐moderate idiopathic pulmonary fibrosis: Comparison of three currently available therapeutic regimens",
"author": "Fiorucci F.",
"first-page": "105",
"issue": "2",
"journal-title": "European Review for Medical and Pharmacological Sciences",
"key": "e_1_2_13_9_1",
"volume": "12",
"year": "2008"
},
{
"DOI": "10.1007/s00330-020-07033-y",
"article-title": "Chest CT score in COVID‐19 patients: Correlation with disease severity and short‐term prognosis",
"author": "Francone M.",
"doi-asserted-by": "crossref",
"first-page": "6808",
"issue": "12",
"journal-title": "European Radiology",
"key": "e_1_2_13_10_1",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2020.01518",
"article-title": "Targeting the NLRP3 inflammasome in severe COVID‐19",
"author": "Freeman T. L.",
"doi-asserted-by": "crossref",
"first-page": "1518",
"journal-title": "Frontiers in Immunology",
"key": "e_1_2_13_11_1",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1016/S2213-2600(20)30225-3",
"article-title": "Pulmonary fibrosis and COVID‐19: The potential role for antifibrotic therapy",
"author": "George P. M.",
"doi-asserted-by": "crossref",
"first-page": "807",
"issue": "8",
"journal-title": "The Lancet Respiratory Medicine",
"key": "e_1_2_13_12_1",
"volume": "8",
"year": "2020"
},
{
"article-title": "Effect of COVID‐19 on the organs",
"author": "Jain U.",
"first-page": "1",
"issue": "8",
"journal-title": "Cureus",
"key": "e_1_2_13_13_1",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1111/eci.13404",
"article-title": "Prognostic implications of neutrophil‐lymphocyte ratio in COVID‐19",
"author": "Jimeno S.",
"doi-asserted-by": "crossref",
"first-page": "e13404",
"journal-title": "European Journal of Clinical Investigation",
"key": "e_1_2_13_14_1",
"volume": "51",
"year": "2021"
},
{
"article-title": "The pathology of severe COVID‐19‐related lung damage: Mechanistic and therapeutic implications",
"author": "Kommoss F. K.",
"first-page": "500",
"issue": "29",
"journal-title": "Deutsches Ärzteblatt International",
"key": "e_1_2_13_15_1",
"volume": "117",
"year": "2020"
},
{
"DOI": "10.1016/j.jrid.2020.07.003",
"article-title": "Semi‐quantitative analysis for the dynamic chest CT imaging features from onset to recovery in severe and critical COVID‐19",
"author": "Liu R.",
"doi-asserted-by": "crossref",
"first-page": "114",
"issue": "3",
"journal-title": "Radiology of Infectious Diseases",
"key": "e_1_2_13_16_1",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1080/08820139.2020.1789655",
"article-title": "Successful treatment of Covid‐19 associated cytokine release syndrome with colchicine. A case report and review of literature",
"author": "Mansouri N.",
"doi-asserted-by": "crossref",
"first-page": "884",
"issue": "8",
"journal-title": "Immunological Investigations",
"key": "e_1_2_13_17_1",
"volume": "50",
"year": "2021"
},
{
"DOI": "10.1183/13993003.01217-2020",
"article-title": "Abnormal pulmonary function in COVID‐19 patients at time of hospital discharge",
"author": "Mo X.",
"doi-asserted-by": "crossref",
"first-page": "2001217",
"issue": "6",
"journal-title": "European Respiratory Journal",
"key": "e_1_2_13_18_1",
"volume": "55",
"year": "2020"
},
{
"DOI": "10.1002/jcph.1645",
"article-title": "Can colchicine as an old anti‐inflammatory agent be effective in COVID‐19?",
"author": "Nasiripour S.",
"doi-asserted-by": "crossref",
"first-page": "828",
"journal-title": "Journal of Clinical Pharmacology",
"key": "e_1_2_13_19_1",
"volume": "60",
"year": "2020"
},
{
"DOI": "10.1148/radiol.2020200370",
"article-title": "Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID‐19) pneumonia",
"author": "Pan F.",
"doi-asserted-by": "crossref",
"first-page": "715",
"journal-title": "Radiology",
"key": "e_1_2_13_20_1",
"volume": "295",
"year": "2020"
},
{
"DOI": "10.1055/s-0038-1636915",
"article-title": "Procoagulant effects of low‐level platelet activation and its inhibition by colchicine",
"author": "Reddel C. J.",
"doi-asserted-by": "crossref",
"first-page": "723",
"issue": "04",
"journal-title": "Thrombosis and Haemostasis",
"key": "e_1_2_13_21_1",
"volume": "47",
"year": "2018"
},
{
"article-title": "Colchicine acutely suppresses the NLRP3 Inflammasome in acute coronary syndrome patients monocytes",
"author": "Robertson S.",
"first-page": "A13715",
"issue": "3",
"journal-title": "Circulation",
"key": "e_1_2_13_22_1",
"volume": "132",
"year": "2015"
},
{
"DOI": "10.1111/jcpt.13251",
"article-title": "Neutrophil Elastase inhibitor (Sivelestat), may be a promising therapeutic option for Management of Acute Lung Injury/acute respiratory distress syndrome or disseminated intravascular coagulation in COVID‐19",
"author": "Sahebnasagh A.",
"doi-asserted-by": "crossref",
"first-page": "1515",
"journal-title": "Journal of Clinical Pharmacy and Therapeutics",
"key": "e_1_2_13_23_1",
"volume": "45",
"year": "2020"
},
{
"DOI": "10.1155/2020/8865954",
"article-title": "A case control study to evaluate the impact of colchicine on patients admitted to the hospital with moderate to severe COVID‐19 infection",
"author": "Sandhu T.",
"doi-asserted-by": "crossref",
"first-page": "8865954",
"journal-title": "Canadian Journal of Infectious Diseases and Medical Microbiology",
"key": "e_1_2_13_24_1",
"volume": "2020",
"year": "2020"
},
{
"DOI": "10.1136/annrheumdis-2020-217712",
"article-title": "Association between treatment with colchicine and improved survival in a single‐Centre cohort of adult hospitalised patients with COVID‐19 pneumonia and acute respiratory distress syndrome",
"author": "Scarsi M.",
"doi-asserted-by": "crossref",
"first-page": "1286",
"issue": "10",
"journal-title": "Annals of the Rheumatic Diseases",
"key": "e_1_2_13_25_1",
"volume": "79",
"year": "2020"
},
{
"DOI": "10.3109/09546634.2015.1116680",
"article-title": "Effects of colchicine treatment on mean platelet volume and the inflammatory markers in recurrent aphthous stomatitis",
"author": "Seçkin H. Y.",
"doi-asserted-by": "crossref",
"first-page": "389",
"issue": "4",
"journal-title": "Journal of Dermatological Treatment",
"key": "e_1_2_13_26_1",
"volume": "27",
"year": "2016"
},
{
"DOI": "10.1093/rheumatology/kex453",
"article-title": "Update on colchicine, 2017",
"author": "Slobodnick A.",
"doi-asserted-by": "crossref",
"first-page": "i4",
"issue": "1",
"journal-title": "Rheumatology",
"key": "e_1_2_13_27_1",
"volume": "57",
"year": "2018"
},
{
"DOI": "10.1136/gutjnl-2020-320891",
"article-title": "SARS‐CoV‐2 induced diarrhoea as onset symptom in patient with COVID‐19",
"author": "Song Y.",
"doi-asserted-by": "crossref",
"first-page": "1143",
"issue": "6",
"journal-title": "Gut",
"key": "e_1_2_13_28_1",
"volume": "69",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.16349",
"article-title": "Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID‐19: A randomized clinical trial",
"author": "Spinner C. D.",
"doi-asserted-by": "crossref",
"first-page": "1048",
"issue": "11",
"journal-title": "JAMA",
"key": "e_1_2_13_29_1",
"volume": "324",
"year": "2020"
},
{
"article-title": "Efficacy of colchicine in non‐hospitalized patients with COVID‐19",
"author": "Tardif J.‐C.",
"journal-title": "Medrxiv",
"key": "e_1_2_13_30_1",
"year": "2021"
},
{
"DOI": "10.1161/JAHA.120.018993",
"article-title": "Colchicine inhibits neutrophil extracellular trap formation in patients with acute coronary syndrome after percutaneous coronary intervention",
"author": "Vaidya K.",
"doi-asserted-by": "crossref",
"first-page": "e018993",
"issue": "1",
"journal-title": "Journal of the American Heart Association",
"key": "e_1_2_13_31_1",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1002/1529-0131(200111)44:11<2686::AID-ART448>3.0.CO;2-H",
"article-title": "A double‐blind trial of colchicine in Behçet's syndrome",
"author": "Yurdakul S.",
"doi-asserted-by": "crossref",
"first-page": "2686",
"issue": "11",
"journal-title": "Arthritis and Rheumatism",
"key": "e_1_2_13_32_1",
"volume": "44",
"year": "2001"
},
{
"article-title": "Discovering and identifying New York heart association classification from electronic health records",
"author": "Zhang R.",
"first-page": "5",
"issue": "2",
"journal-title": "BMC Medical Informatics and Decision Making",
"key": "e_1_2_13_33_1",
"volume": "18",
"year": "2018"
}
],
"reference-count": 32,
"references-count": 32,
"relation": {},
"score": 1,
"short-container-title": [
"Phytotherapy Research"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology"
],
"subtitle": [],
"title": [
"Efficacy and safety of colchicine treatment in patients with\n COVID\n ‐19: A prospective, multicenter, randomized clinical trial"
],
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy"
}
