Recombinant human plasma gelsolin (rhu-pGSN) is a biologic therapy that functions as a host-directed anti-inflammatory and immunomodulatory agent.
Jul 25 2022 |
et al., Open Forum Infectious Diseases, doi:10.1093/ofid/ofac357 | Adjunctive Recombinant Human Plasma Gelsolin for Severe Coronavirus Disease 2019 Pneumonia |
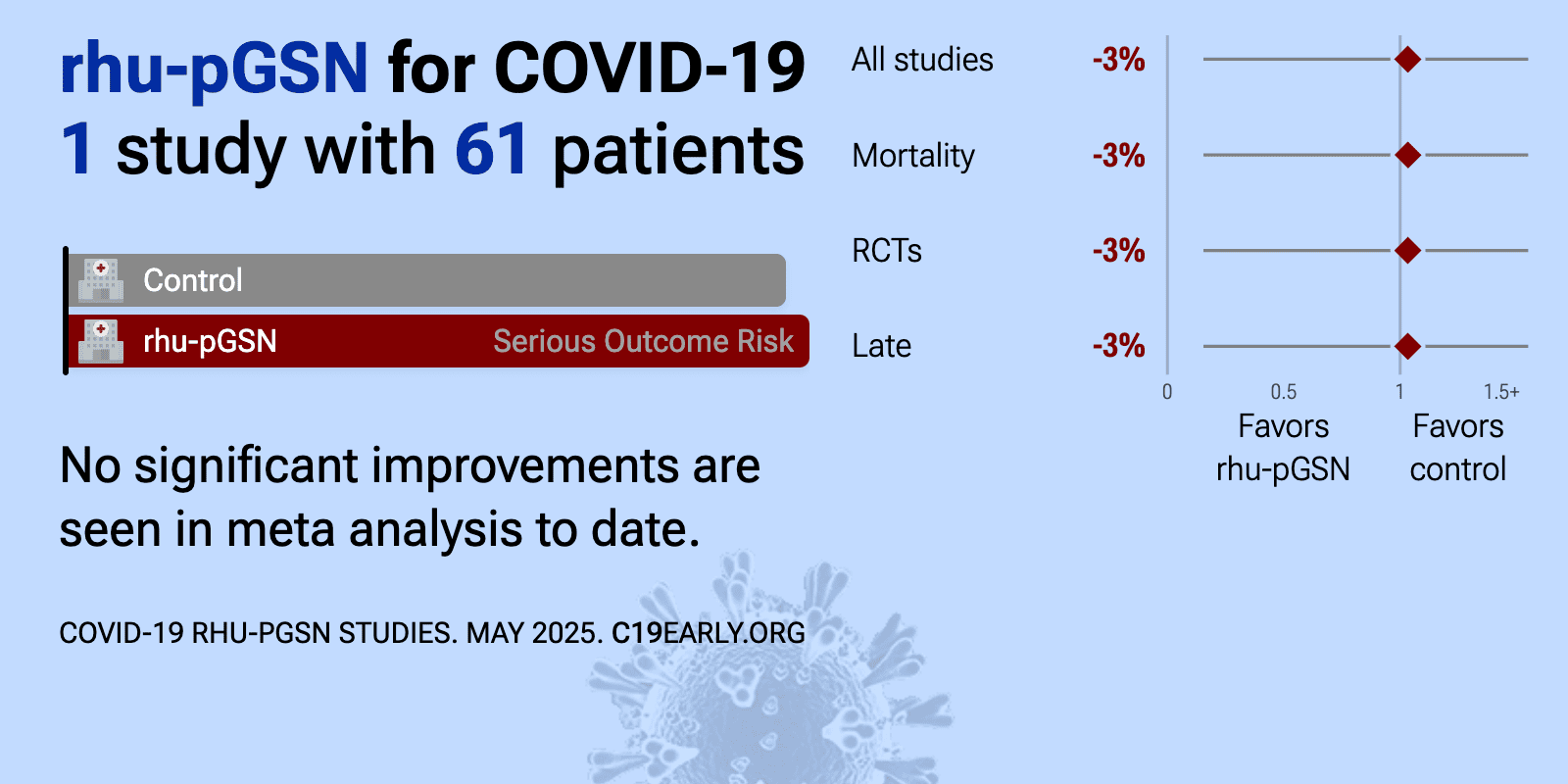
| 3% higher mortality (p=1), 48% lower ventilation (p=0.47), and 29% higher progression (p=0.73). RCT 61 hospitalized patients with COVID-19 pneumonia showing no significant difference in survival without major organ support with recombinant human plasma gelsolin (rhu-pGSN). This double-blind, placebo-controlled trial evaluated rhu-pG.. | ||