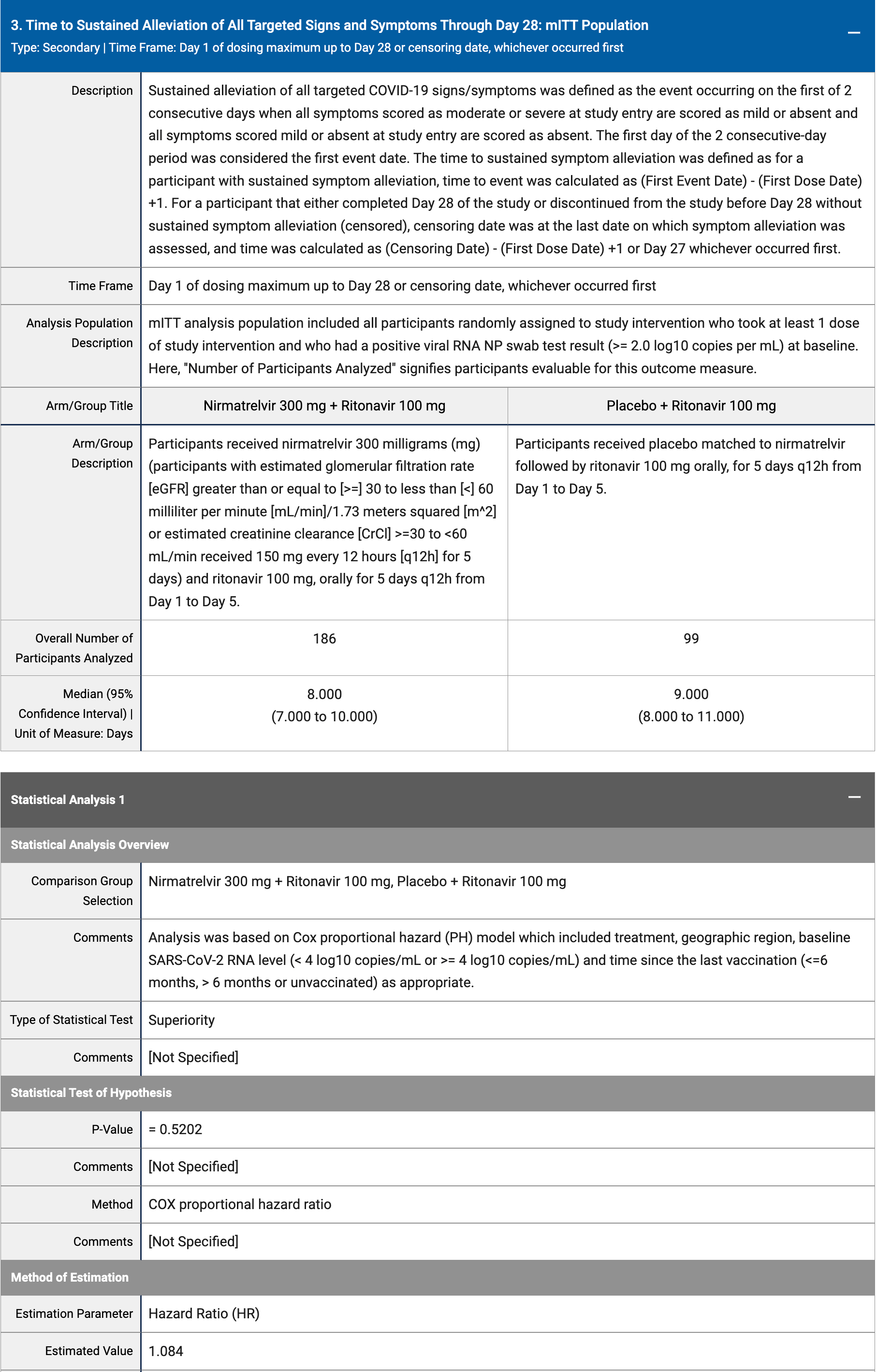
|
risk of no recovery, 7.9% lower, HR 0.92, p = 0.52, treatment 186, control 99, inverted to make HR<1 favor treatment, Cox proportional hazards, day 28.
|
|
relative reduction in viral load, 18.2% better, RR 0.82, p < 0.001, treatment 218, control 113, day 5.
|
|
risk of no viral clearance, 19.0% lower, HR 0.81, p = 0.07, treatment 223, control 115, inverted to make HR<1 favor treatment, time to two consecutive negative RAT results at least 24 hours apart, Cox proportional hazards, day 28.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |