Comparison Study of the Bio-Plex and Meso Scale Multiplexed SARS-CoV-2 Serology Assays Reveals Evidence of Diminished Host Antibody Responses to SARS-CoV-2 after Monoclonal Antibody Treatment
et al., Pathogens and Immunity, 10.20411/pai.v9i2.715, Aug 2024
25th treatment shown to reduce risk in
May 2021, now with p = 0.00049 from 22 studies, recognized in 11 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
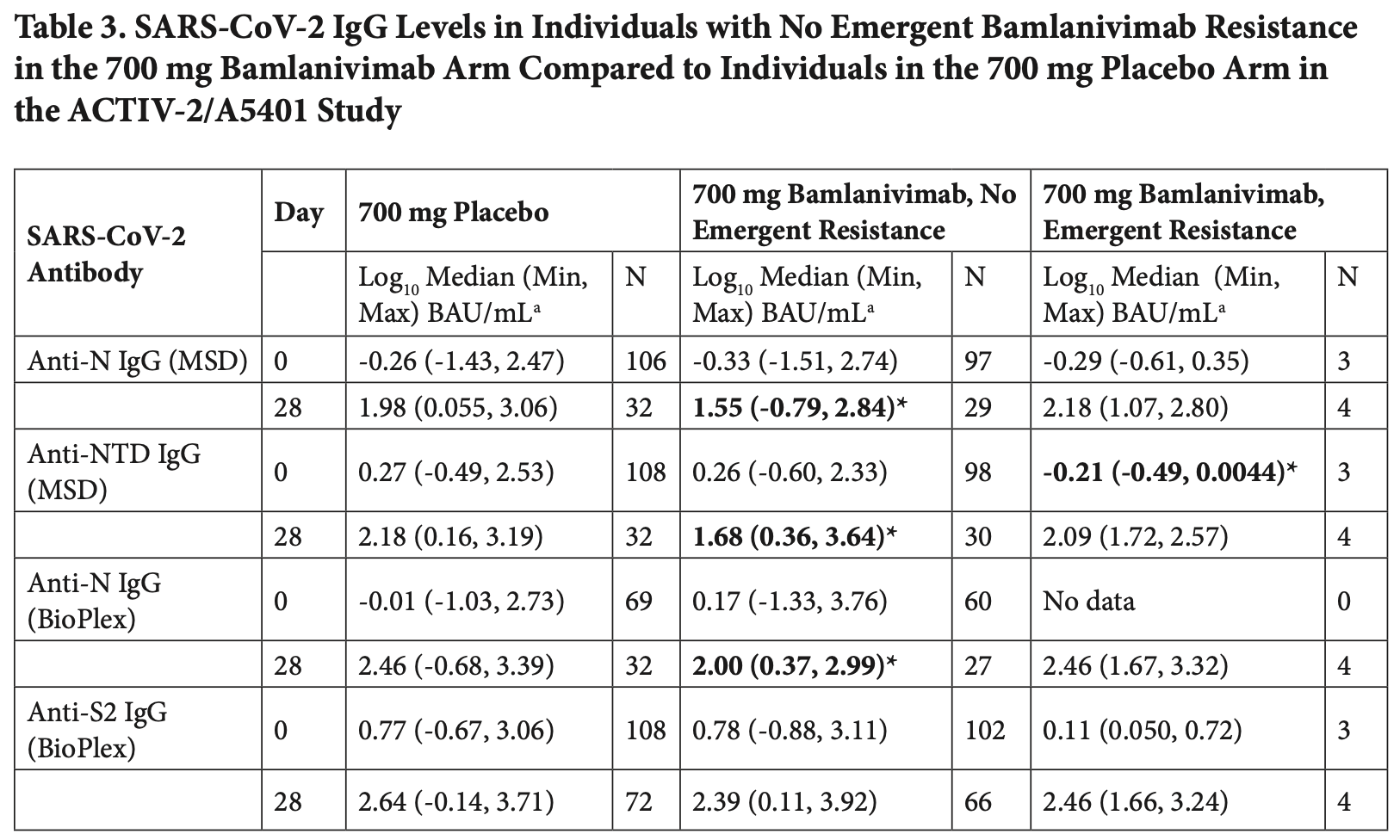
Comparison of two multiplex serology assays for detecting SARS-CoV-2 antibodies in 455 samples from 304 participants in the ACTIV-2/A5401 trial of bamlanivimab for non-hospitalized COVID-19 patients. Authors found diminished host antibody responses in bamlanivimab-treated participants compared to placebo. For anti-N IgG at day 28, the bamlanivimab group (700 mg) showed a median of 1.55 log BAU/mL compared to 1.98 log BAU/mL in the placebo group, representing approximately a 2.7-fold decrease. Similar trends were observed for other host-derived antibodies (anti-NTD, anti-S2) and time points. Authors attribute this to reduced viral antigen exposure due to mAb treatment. The results suggest mAb treatment may impact the development of natural immunity.
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
Parikh et al., 15 Aug 2024, peer-reviewed, 16 authors.
Comparison Study of the Bio-Plex and Meso Scale Multiplexed SARS-CoV-2 Serology Assays Reveals Evidence of Diminished Host Antibody Responses to SARS-CoV-2 after Monoclonal Antibody Treatment
doi:10.20411/pai.v9i2.715
Background: Assessing the breadth and duration of antigen-specific binding antibodies provides valuable information for evaluating interventions to treat or prevent SARS-CoV-2 infection. Multiplex immunoassays are a convenient method for rapid measurement of antibody responses but can sometimes provide discordant results, and antibody positive percent agreement for COVID-19 diagnosis can vary depending on assay type, disease severity, and population sampled. Therefore, we compared two assays marked for research applications, MSD and Bio-Plex Pro, to evaluate qualitative interpretation of serostatus and quantitative detection of antibodies of varying isotypes (IgG, IgM, and IgA) against receptor binding domain (RBD) and nucleocapsid (N) antigens. Methods: Specimens from ACTIV-2/A5401, a placebo-controlled clinical trial of the SARS-CoV-2 monoclonal antibody (mAb) bamlanivimab to prevent COVID-19 disease progression, were used to evaluate the concordance of the Bio-Rad Bio-Plex Pro Human SARS-CoV-2 Serology Assay and the Meso Scale Discovery (MSD) V-PLEX COVID-19 Panel 1 serology assay in detecting and quantifying IgG, IgA, and IgM binding anti-SARS-CoV-2 antibody responses against the RBD and N antigens. Data were disaggregated by study arm, bamlanivimab dose, days post-enrollment, and presence of emerging resistance.
Results: We observed 90.5% (412 of 455 tests) concordance for anti-RBD IgG and 87% (396 of 455) concordance for anti-N IgG in classifying samples as negative or positive based on assay-defined cutoffs. Antibody levels converted to the WHO standard BAU/mL were significantly correlated for all isotypes (IgG, IgM, and IgA) and SARS-CoV-2 antigen targets (RBD and N) tested that were common between the two assays (Spearman r 0.65 to 0.92, P < 0.0001). Both assays uncovered evidence of diminished host-derived IgG immune responses in participants treated with bamlanivimab compared to placebo. Assessment of immune responses in the four individuals treated with the 700 mg of bamlanivimab with emerging mAb resistance demonstrated a stronger anti-N IgG response (MSD) at day 28 (median 2.18 log BAU/mL) compared to participants treated with bamlanivimab who did not develop resistance (median 1.55 log BAU/mL). Conclusions: These data demonstrate the utility in using multiplex immunoassays for characterizing the immune responses with and without treatment in a study population and provide evidence that monoclonal antibody treatment in acute COVID-19 may have a modest negative impact on development of host IgG responses.
AUTHOR STATEMENTS U.M.P. and S.F.S. designed the study and wrote the manuscript. A.L.H., D.M., K.C.G., M.C.C., R.D., and C.M. conducted experiments and performed analysis. J.W.M., P.K., A.L.L., J.S.C., J.J.E., K.W.C., D.M.S., and J.Z.L. contributed to study interpretation and manuscript review. K.W.C. and D.M.S. conducted the study from which samples were obtained.
References
Alter, Yu, Liu, Chandrashekar, Borducchi et al., Immunogenicity of Ad26.COV2.S vaccine against SARS-CoV-2 variants in humans, Nature, doi:10.1038/s41586-021-03681-2
Bio-Rad, Bio-Plex Pro Human IgA and IgM SARS-CoV-2 Serology Assays Protocol, Bulletin
Blom, Marking, Havervall, Norin, Gordon et al., Immune responses after omicron infection in triple-vaccinated healthcare workers with and without previous SARS-CoV-2 infection, Lancet Infect Dis, doi:10.1016/S1473-3099(22)00362-0
Bolton, Chaudhury, Dutta, Gregory, Pierson et al., Comparison of ELISA with electro-chemiluminescence technology for the qualitative and quantitative assessment of serological responses to vaccination, Malar J, doi:10.1186/s12936-020-03225-5
Bozorgmehr, Mashhouri, Rosero, Xu, Shahbaz et al., Galectin-9, a Player in Cytokine Release Syndrome and a Surrogate Diagnostic Biomarker in SARS-CoV-2 Infection, mBio, doi:10.1128/mBio.00384-21
Chew, Moser, Daar, Wohl, Li et al., Antiviral and clinical activity of bamlanivimab in a randomized trial of non-hospitalized adults with COVID-19, Nat Commun, doi:10.1038/s41467-022-32551-2
Choudhary, Chew, Deo, Flynn, Regan et al., Emergence of SARS-CoV-2 escape mutations during Bamlanivimab therapy in a phase II randomized clinical trial, Nat Microbiol, doi:10.1038/s41564-022-01254-1
Cox, Gao, Quantitative Bio-Plex Pro Human IgG SARS-CoV-2 Serology Assays Using a Standard Curve, Bio-Rad Laboratories Technical Bulletin
Dai, Gao, Viral targets for vaccines against COVID-19, Nat Rev Immunol, doi:10.1038/s41577-020-00480-0
Fox, Geppert, Dinnes, Scandrett, Bigio et al., Antibody tests for identification of current and past infection with SARS-CoV-2, Cochrane Database Syst Rev, doi:10.1002/14651858.CD013652.pub2
Grunau, Asamoah-Boaheng, Lavoie, Karim, Kirkham et al., A Higher Antibody Response Is Generated With a 6-to 7-Week (vs Standard) Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vaccine Dosing Interval, Clin Infect Dis, doi:10.1093/cid/ciab938
Grzelak, Temmam, Planchais, Demeret, Tondeur et al., A comparison of four serological assays for detecting anti-SARS-CoV-2 antibodies in human serum samples from different populations, Sci Transl Med, doi:10.1126/scitranslmed.abc3103
Ige, Hamada, Steinhardt, Iriemenam, Uwandu et al., Validation of Commercial SARS-CoV-2 Immunoassays in a Nigerian Population, Microbiol Spectr, doi:10.1128/Spectrum.00680-21
Infantino, Pieri, Nuccetelli, Grossi, Lari et al., The WHO International Standard for COVID-19 serological tests: towards harmonization of anti-spike assays, Int Immunopharmacol, doi:10.1016/j.intimp.2021.108095
Iyer, Jones, Nodoushani, Kelly, Becker et al., Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients, Sci Immunol, doi:10.1126/sciimmu-nol.abe0367
Jacob-Dolan, Feldman, Mcmahan, Yu, Zahn et al., Coronavirus-Specific Antibody Cross Reactivity in Rhesus Macaques Following SARS-CoV-2 Vaccination and Infection, J Virol, doi:10.1128/JVI.00117-21
Jones, Brown-Augsburger, Corbett, Westendorf, Davies et al., The neutralizing antibody, LY-CoV555, protects against SARS-CoV-2 infection in nonhuman primates, Sci Transl Med, doi:10.1126/scitranslmed.abf1906
Karaba, Johnston, Aytenfisu, Akinde, Eby et al., A Fourth Dose of COVID-19 Vaccine Does Not Induce Neutralization of the Omicron Variant Among Solid Organ Transplant Recipients With Suboptimal Vaccine Response, Transplantation, doi:10.1097/TP.0000000000004140
Kenny, Negi, Reilly, Garcia-Leon, Alalwan et al., cohort s. Performance and validation of an adaptable multiplex assay for detection of serologic response to SARS-CoV-2 infection or vaccination, J Immunol Methods, doi:10.1016/j.jim.2022.113345
Kweon, Lim, Kim, Kim, Choi et al., Antibody kinetics and serologic profiles of SARS-CoV-2 infection using two serologic assays, PLoS One, doi:10.1371/journal.pone.0240395
Marra, Kobayashi, Suzuki, Alsuhaibani, Tofaneto et al., Short-term effectiveness of COVID-19 vaccines in immunocompromised patients: A systematic literature review and meta-analysis, J Infect, doi:10.1016/j.jinf.2021.12.035
Moser, Li, Eron, Aga, Daar et al., Predictors of SARS-CoV-2 RNA From Nasopharyngeal Swabs and Concordance With Other Compartments in Nonhospitalized Adults With Mild to Moderate COVID-19, Open Forum Infect Dis, doi:10.1093/ofid/ofac618
Ong, Fragkou, Schweitzer, Chemaly, Moschopoulos et al., How to interpret and use COVID-19 serology and immunology tests, Clin Microbiol Infect, doi:10.1016/j.cmi.2021.05.001
Saraf, Zhu, Shrestha, Bonny, Baker et al., Differential antibody production by symptomatology in SARS-CoV-2 convalescent individuals, PLoS One, doi:10.1371/journal.pone.0264298
Sohaei, Ulndreaj, Mathew, Campbell, Stengelin et al., Sensitive Serology Measurements in the Saliva of Individuals with COVID-19 Symptoms Using a Multiplexed Immunoassay, J Appl Lab Med, doi:10.1093/jalm/jfac073
Ward, Mullins, Pickett, Merrill, Ruiz et al., Performance of 4 Automated SARS-CoV-2 Serology Assay Platforms in a Large Cohort Including Susceptible COVID-19-Negative and COVID-19-Positive Patients, J Appl Lab Med, doi:10.1093/jalm/jfab014
Wheeler, Shurin, Yost, Anderson, Pinto et al., Differential Antibody Response to mRNA COVID-19 Vaccines in Healthy Subjects, Microbiol Spectr, doi:10.1128/Spectrum.00341-21
Woldemeskel, Garliss, Aytenfisu, Johnston, Beck et al., SARS-CoV-2-specific immune responses in boosted vaccine recipients with breakthrough infections during the Omicron variant surge, JCI Insight, doi:10.1172/jci.insight.159474
Yang, Nielsen, Hoh, Roltgen, Wirz et al., Shared B cell memory to coronaviruses and other pathogens varies in human age groups and tissues, Science, doi:10.1126/science.abf6648
Yun, Ryu, Jang, Bae, Yoo et al., Comparison of SARS-CoV-2 Antibody Responses and Seroconversion in COVID-19 Patients Using Twelve Commercial Immunoassays, Ann Lab Med, doi:10.3343/alm.2021.41.6.577
Zhang, Poorbaugh, Dougan, Chen, Gottlieb et al., Endogenous Antibody Responses to SARS-CoV-2 in Patients With Mild or Moderate COVID-19 Who Received Bamlanivimab Alone or Bamlanivimab and Etesevimab Together, Front Immunol, doi:10.3389/fimmu.2021.790469
Zhou, Zhang, Xie, Wu, Advancements in detection of SARS-CoV-2 infection for confronting COVID-19 pandemics, Lab Invest, doi:10.1038/s41374-021-00663-w
