CX3CR1–TLR4 Axis as a Shared Neuroimmune Target in COVID-19 and Epilepsy: Integrative Transcriptomics and Gabapentin Repositioning
et al., Biomedicines, doi:10.3390/biomedicines13092133, Aug 2025
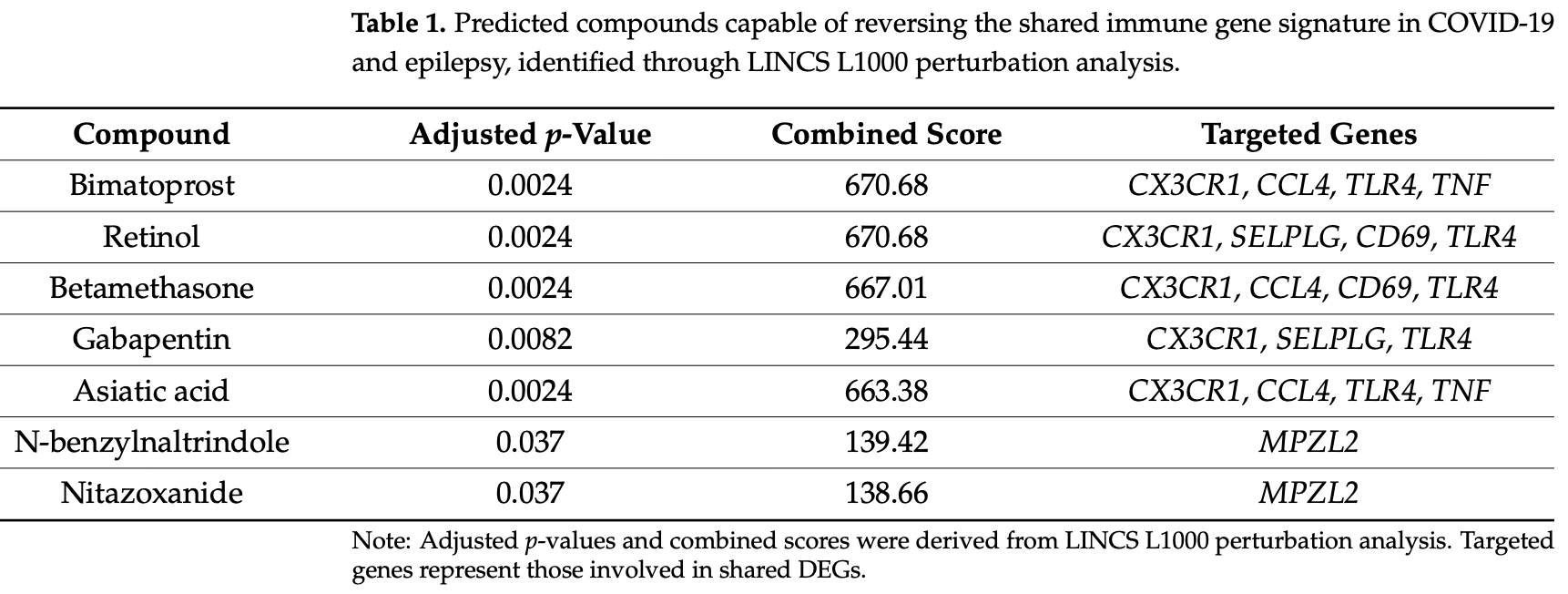
In silico study showing that gabapentin may be beneficial for COVID-19 treatment through shared neuroinflammatory pathways with epilepsy. Authors performed integrative transcriptomic analysis of COVID-19 peripheral blood mononuclear cells and epilepsy hippocampal tissue, identifying 25 shared differentially expressed genes including upregulated CX3CR1, TLR4, TNF, and CCL3 involved in cytokine signaling and immune cell recruitment.
Pan et al., 31 Aug 2025, peer-reviewed, 6 authors.
Contact: ningjeny@126.com (corresponding author), 2024360094@gzhmu.edu.com.
In silico studies are an important part of preclinical research, however results may be very different in vivo.
CX3CR1–TLR4 Axis as a Shared Neuroimmune Target in COVID-19 and Epilepsy: Integrative Transcriptomics and Gabapentin Repositioning
Biomedicines, doi:10.3390/biomedicines13092133
Introduction: Neuroinflammation is a common pathological hallmark of Coronavirus Disease 2019 (COVID-19) and epilepsy; however, their shared immunogenomic mechanisms remain poorly defined. This study explores shared immune-inflammatory transcriptomic signatures and identifies potential repositioning therapeutics. Methods: We integrated single-cell RNA-seq data from peripheral blood mononuclear cells (PBMCs) of COVID-19 patients and healthy donors (GSE149689), and bulk RNA-seq data from hippocampal tissue of patients with Temporal Lobe Epilepsy with Hippocampal Sclerosis (TLE-HS) and healthy controls (GSE256068). Common Differentially Expressed Genes (DEGs) were identified and subjected to GO/KEGG enrichment, a PPI network, hub gene detection (cytoHubba), and transcriptional regulation analysis (ENCODE-based TF/miRNA networks). Drug repositioning was performed using the LINCS L1000 database. Results: We identified 25 DEGs shared across datasets, including 22 upregulated genes enriched in cytokine-cytokine receptor interaction, NF-κB, and Toll-like receptor pathways. PPI analysis revealed a CX3CR1-TLR4-centered immune module. Gabapentin emerged as a promising repositioning candidate with potential to downregulate CX3CR1, TLR4, and selectin P ligand (SELPLG). Receiver Operating Characteristic (ROC) analysis confirmed the diagnostic value of these targets (AUC > 0.90 in epilepsy). A mechanistic model was proposed to illustrate Gabapentin's dual action on microglial polarization and cytokine suppression. Conclusions: Our results reveal a shared CX3CR1-TLR4-NF-κB inflammatory axis in COVID-19 and epilepsy, supporting Gabapentin as a potential dual-action immunomodulator. These findings reveal a previously underappreciated immunomodulatory role for Gabapentin, providing mechanistic rationale for its repositioning in neuroinflammatory conditions beyond seizure control.
Supplementary Materials: The following supporting information can be downloaded at: https: //www.mdpi.com/article/10.3390/biomedicines13092133/s1 , Supplementary File S1: Common upregulated DEGs with statistical details; Supplementary File S2:
References
Abdelnaser, Alaaeldin, Attya, Fathy, Hepatoprotective potential of gabapentin in cecal ligation and punctureinduced sepsis; targeting oxidative stress, apoptosis, and NF-kB/MAPK signaling pathways, Life Sci, doi:10.1016/j.lfs.2023.121562
Ahmad, Shoaib, Ahsan, Deng, Ma et al., Microglial IL-10 and beta-endorphin expression mediates gabapentinoids antineuropathic pain, Brain Behav. Immun, doi:10.1016/j.bbi.2021.04.007
Ali, Chugh, Ekdahl, Role of fractalkine-CX3CR1 pathway in seizure-induced microglial activation, neurodegeneration, and neuroblast production in the adult rat brain, Neurobiol. Dis, doi:10.1016/j.nbd.2014.11.009
Alves, Gil, Villegas-Salmeron, Salari, Martins-Ferreira et al., Opposing effects of the purinergic P2X7 receptor on seizures in neurons and microglia in male mice, Brain Behav. Immun, doi:10.1016/j.bbi.2024.05.023
Bensken, O'brien, The Significance of the Increased Incidence of New Onset Seizures and Epilepsy After a COVID-19 Infection, Neurology, doi:10.1212/WNL.0000000000201651
Berg, Jobst, Epilepsy and COVID-19's Double-Edged Sword: More Severe Disease and Delayed Epilepsy Care, Neurology, doi:10.1212/WNL.0000000000200367
Chagas, Serfaty, The Influence of Microglia on Neuroplasticity and Long-Term Cognitive Sequelae in Long COVID: Impacts on Brain Development and Beyond, Int. J. Mol. Sci, doi:10.3390/ijms25073819
Chakraborty, Gonzalez, Edwards, Mallajosyula, Buzzanco et al., Proinflammatory IgG Fc structures in patients with severe COVID-19, Nat. Immunol, doi:10.1038/s41590-020-00828-7
Cibrian, Sanchez-Madrid, From activation marker to metabolic gatekeeper, Eur. J. Immunol, doi:10.1002/eji.201646837
Cothran, Kellman, Singh, Beck, Powell et al., A brewing storm: The neuropsychological sequelae of hyperinflammation due to COVID-19, Brain Behav. Immun, doi:10.1016/j.bbi.2020.06.008
Croce, Gao, Wang, Mooroka, Sakuma et al., Myeloid-related protein-8/14 is critical for the biological response to vascular injury, Circulation, doi:10.1161/CIRCULATIONAHA.108.814582
Deng, Li, Zhang, Xian-Yu, Tao et al., Effectiveness and safety of single anti-seizure medication as adjunctive therapy for drug-resistant focal epilepsy based on network meta-analysis, Front. Pharmacol, doi:10.3389/fphar.2025.1500475
Diebold, Vietzen, Heinzel, Haindl, Herz et al., Natural killer cell functional genetics and donor-specific antibody-triggered microvascular inflammation, Am. J. Transplant, doi:10.1016/j.ajt.2023.12.005
Dress, Ginhoux, Monocytes and macrophages in severe COVID-19-Friend, foe or both?, Immunol. Cell Biol, doi:10.1111/imcb.12464
Egervari, Thomas, Lobrinus, Kuhlmann, Bruck et al., Neuropathology associated with SARS-CoV-2 infection, Lancet, doi:10.1016/S0140-6736(21)00095-7
El-Kattan, Saeed, Khattab, Maksoud, Eldein et al., Gabapentin-Induced Sub-Chronic Neurotoxicity in Rats and the Protective Role of Alpha-Tocopherol, doi:10.5146/tjpath.2025.14236
Feng, Qiao, Xia, Yu, Lang et al., ultrasmall and catalytic potassium calcium hexacyanoferrate for neuroprotection and temporal lobe epilepsy treatment, Sci. Bull, doi:10.1016/j.scib.2025.02.036
Garcia, Miller, Norwood, Dorin, Grayson et al., Gabapentin improves parosmia after COVID-19 infection, Int. Forum Allergy Rhinol, doi:10.1002/alr.23117
Goonewardena, Chen, Tate, Grushko, Damodaran et al., Monocyte-Mediated Thrombosis Linked to Circulating Tissue Factor and Immune Paralysis in COVID-19, Arterioscler. Thromb. Vasc. Biol, doi:10.1161/ATVBAHA.122.318721
Guangzhou, None
Kaushik, Bhandari, Kuhad, TLR4 as a therapeutic target for respiratory and neurological complications of SARS-CoV-2, Expert. Opin. Ther. Targets, doi:10.1080/14728222.2021.1918103
Kim, Lee, Kim, Park, Kang, Distinct Roles of CK2-and AKT-Mediated NF-kappaB Phosphorylations in Clasmatodendrosis (Autophagic Astroglial Death) within the Hippocampus of Chronic Epilepsy Rats, Antioxidants, doi:10.3390/antiox12051020
Klein, Kaminski, Koepp, Loscher, New epilepsy therapies in development, Nat. Rev. Drug Discov, doi:10.1038/s41573-024-00981-w
Kohler, Bohm, Rolfs, Egger, Hornemann et al., NF-kappaB/RelA and Nrf2 cooperate to maintain hepatocyte integrity and to prevent development of hepatocellular adenoma, J. Hepatol, doi:10.1016/j.jhep.2015.08.033
Kuehn, Growing Role of Gabapentin in Opioid-Related Overdoses Highlights Misuse Potential and Off-label Prescribing Practices, JAMA, doi:10.1001/jama.2022.13659
Kyriatzis, Bernard, Bole, Khrestchatisky, Ferhat, In the Rat Hippocampus, Pilocarpine-Induced Status Epilepticus Is Associated with Reactive Glia and Concomitant Increased Expression of CD31, PDGFRbeta, and Collagen IV in Endothelial Cells and Pericytes of the Blood-Brain Barrier, Int. J. Mol. Sci, doi:10.3390/ijms25031693
Lee, Jun, Kim, Park, Intrathecal gabapentin increases interleukin-10 expression and inhibits pro-inflammatory cytokine in a rat model of neuropathic pain, J. Korean Med. Sci, doi:10.3346/jkms.2013.28.2.308
Lempriere, High rate of epilepsy in young individuals who died with COVID-19, Nat. Rev. Neurol, doi:10.1038/s41582-021-00610-9
Liang, Sri Hari, Day, Patel, Pharmacological elevation of glutathione inhibits status epilepticus-induced neuroinflammation and oxidative injury, Redox Biol, doi:10.1016/j.redox.2024.103168
Librizzi, Noe, Vezzani, De Curtis, Ravizza, Seizure-induced brain-borne inflammation sustains seizure recurrence and blood-brain barrier damage, Ann. Neurol, doi:10.1002/ana.23567
Liu, Xu, Dai, Tan, Mao et al., Dynorphin activation of kappa opioid receptor promotes microglial polarization toward M2 phenotype via TLR4/NF-kappaB pathway, Cell Biosci, doi:10.1186/s13578-020-00387-2
Lu, Xiong, Liu, Liu, Yang et al., New onset acute symptomatic seizure and risk factors in coronavirus disease 2019: A retrospective multicenter study, Epilepsia, doi:10.1111/epi.16524
Majeed, Rudnick, Bonkovsky, Review of Antiseizure Medications for Adults With Epilepsy, JAMA, doi:10.1001/jama.2022.10594
Mcwilliam, Samuel, Alkufri, Neuropathic pain post-COVID-19: A case report, BMJ Case Rep, doi:10.1136/bcr-2021-243459
Morales-Chacon, Vertical transmission of SARS-CoV-2. Impact on the nervous system, Rev. Med. Inst. Mex. Seguro Soc, doi:10.24875/RMIMSS.M20000132
Mowry, Peaden, Stern, Biancardi, TLR4 and AT1R mediate blood-brain barrier disruption, neuroinflammation, and autonomic dysfunction in spontaneously hypertensive rats, Pharmacol. Res, doi:10.1016/j.phrs.2021.105877
Nikbakht, Mohammadkhanizadeh, Mohammadi, How does the COVID-19 cause seizure and epilepsy in patients? The potential mechanisms, Mult. Scler. Relat. Disord, doi:10.1016/j.msard.2020.102535
Owens, Garcia, Mochizuki, Chang, Reyes et al., Evidence for Innate and Adaptive Immune Responses in a Cohort of Intractable Pediatric Epilepsy Surgery Patients, Front. Immunol, doi:10.3389/fimmu.2019.00121
Qureshi, Mehler, Epigenetics and therapeutic targets mediating neuroprotection, Brain Res, doi:10.1016/j.brainres.2015.07.034
Rissardo, Medeiros Araujo De Matos, Fornari Caprara, Gabapentin-Associated Movement Disorders: A Literature Review, Medicines, doi:10.3390/medicines10090052
Rossi, Murta, Auzmendi, Ramos, Early Gabapentin Treatment during the Latency Period Increases Convulsive Threshold, Reduces Microglial Activation and Macrophage Infiltration in the Lithium-Pilocarpine Model of Epilepsy, Pharm, doi:10.3390/ph10040093
Santos De Lima, Issa, Seibert, Davis, Wlodarski et al., Epileptiform activity and seizures in patients with COVID-19, J. Neurol. Neurosurg. Psychiatry, doi:10.1136/jnnp-2020-324337
Soltani, Nasirharandi, Khorvash, Nasirian, Dolatshahi et al., The effectiveness of gabapentin and gabapentin/montelukast combination compared with dextromethorphan in the improvement of COVID-19-related cough: A randomized, controlled clinical trial, Clin. Respir. J, doi:10.1111/crj.13529
Subbarayan, Joly-Amado, Bickford, Nash, CX3CL1/CX3CR1 signaling targets for the treatment of neurodegenerative diseases, Pharmacol. Ther, doi:10.1016/j.pharmthera.2021.107989
Tharakan, Kallogjeri, Piccirillo, Clinical studies in COVID-related olfactory disorders: Review of an institutional experience, World J. Otorhinolaryngol. Head. Neck Surg, doi:10.1002/wjo2.176
Umpierre, Li, Ayasoufi, Simon, Zhao et al., Microglial P2Y(6) calcium signaling promotes phagocytosis and shapes neuroimmune responses in epileptogenesis, Neuron, doi:10.1016/j.neuron.2024.03.017
Vezzani, Balosso, Ravizza, Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy, Nat. Rev. Neurol, doi:10.1038/s41582-019-0217-x
Villasana-Salazar, Vezzani, Neuroinflammation microenvironment sharpens seizure circuit, Neurobiol. Dis, doi:10.1016/j.nbd.2023.106027
Wang, Han, Wang, Wang, Peng et al., SARS-CoV-2 membrane protein induces neurodegeneration via affecting Golgi-mitochondria interaction, Transl. Neurodegener, doi:10.1186/s40035-024-00458-1
Winneberger, Schols, Lessmann, Randez-Garbayo, Bauer et al., Platelet endothelial cell adhesion molecule-1 is a gatekeeper of neutrophil transendothelial migration in ischemic stroke, Brain Behav. Immun, doi:10.1016/j.bbi.2020.12.026
Yang, Jia, Xiao, Zhao, Lu et al., Baicalin Rescues Cognitive Dysfunction, Mitigates Neurodegeneration, and Exerts Anti-Epileptic Effects Through Activating TLR4/MYD88/Caspase-3 Pathway in Rats, Drug Des. Devel Ther, doi:10.2147/DDDT.S314076
Yang, Xu, Li, Zhang, Xu et al., Gabapentin reduces CX3CL1 signaling and blocks spinal microglial activation in monoarthritic rats, Mol. Brain, doi:10.1186/1756-6606-5-18
Yeo, Kim, Ryu, Seo, Lee et al., The roles of fractalkine/CX3CR1 system in neuronal death following pilocarpine-induced status epilepticus, J. Neuroimmunol, doi:10.1016/j.jneuroim.2011.03.005
Yli-Karjanmaa, Larsen, Fenger, Kristensen, Martin et al., TNF deficiency causes alterations in the spatial organization of neurogenic zones and alters the number of microglia and neurons in the cerebral cortex, Brain Behav. Immun, doi:10.1016/j.bbi.2019.08.195
Yoo, Kim, Jeon, Kim, Song, Risk of COVID-19 Infection and of Severe Complications Among People With Epilepsy: A Nationwide Cohort Study, Neurology, doi:10.1212/WNL.0000000000200195
Zheng, Zhao, Chang, Zhuang, Waimei et al., beta-Sitosterol Alleviates Neuropathic Pain by Affect Microglia Polarization through Inhibiting TLR4/NF-kappaB Signaling Pathway, J. Neuroimmune Pharmacol, doi:10.1007/s11481-023-10091-w
DOI record:
{
"DOI": "10.3390/biomedicines13092133",
"ISSN": [
"2227-9059"
],
"URL": "http://dx.doi.org/10.3390/biomedicines13092133",
"abstract": "<jats:p>Introduction: Neuroinflammation is a common pathological hallmark of Coronavirus Disease 2019 (COVID-19) and epilepsy; however, their shared immunogenomic mechanisms remain poorly defined. This study explores shared immune-inflammatory transcriptomic signatures and identifies potential repositioning therapeutics. Methods: We integrated single-cell RNA-seq data from peripheral blood mononuclear cells (PBMCs) of COVID-19 patients and healthy donors (GSE149689), and bulk RNA-seq data from hippocampal tissue of patients with Temporal Lobe Epilepsy with Hippocampal Sclerosis (TLE-HS) and healthy controls (GSE256068). Common Differentially Expressed Genes (DEGs) were identified and subjected to GO/KEGG enrichment, a PPI network, hub gene detection (cytoHubba), and transcriptional regulation analysis (ENCODE-based TF/miRNA networks). Drug repositioning was performed using the LINCS L1000 database. Results: We identified 25 DEGs shared across datasets, including 22 upregulated genes enriched in cytokine–cytokine receptor interaction, NF-κB, and Toll-like receptor pathways. PPI analysis revealed a CX3CR1–TLR4-centered immune module. Gabapentin emerged as a promising repositioning candidate with potential to downregulate CX3CR1, TLR4, and selectin P ligand (SELPLG). Receiver Operating Characteristic (ROC) analysis confirmed the diagnostic value of these targets (AUC > 0.90 in epilepsy). A mechanistic model was proposed to illustrate Gabapentin’s dual action on microglial polarization and cytokine suppression. Conclusions: Our results reveal a shared CX3CR1–TLR4–NF-κB inflammatory axis in COVID-19 and epilepsy, supporting Gabapentin as a potential dual-action immunomodulator. These findings reveal a previously underappreciated immunomodulatory role for Gabapentin, providing mechanistic rationale for its repositioning in neuroinflammatory conditions beyond seizure control.</jats:p>",
"alternative-id": [
"biomedicines13092133"
],
"author": [
{
"affiliation": [
{
"name": "Department of Neurology, Affiliated Brain Hospital of Guangzhou Medical University, Guangzhou 510370, China"
}
],
"family": "Pan",
"given": "Nannan",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Psychiatry, The Fifth Affiliated Hospital of Sun Yat-sen University, Zhuhai 519000, China"
}
],
"family": "Cao",
"given": "Penghui",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Geriatric Neuroscience Center, Affiliated Brain Hospital of Guangzhou Medical University, Guangzhou 510370, China"
}
],
"family": "Chen",
"given": "Ben",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Neurology, Affiliated Brain Hospital of Guangzhou Medical University, Guangzhou 510370, China"
}
],
"family": "Chen",
"given": "Li",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Neurology, Affiliated Brain Hospital of Guangzhou Medical University, Guangzhou 510370, China"
}
],
"family": "Liao",
"given": "Xuezhen",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-5727-2782",
"affiliation": [
{
"name": "Geriatric Neuroscience Center, Affiliated Brain Hospital of Guangzhou Medical University, Guangzhou 510370, China"
},
{
"name": "Key Laboratory of Neurogenetics and Channelopathies of Guangdong Province and the Ministry of Education of China, Guangzhou Medical University, Guangzhou 511436, China"
}
],
"authenticated-orcid": false,
"family": "Ning",
"given": "Yuping",
"sequence": "additional"
}
],
"container-title": "Biomedicines",
"container-title-short": "Biomedicines",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2025,
9,
1
]
],
"date-time": "2025-09-01T13:27:44Z",
"timestamp": 1756733264000
},
"deposited": {
"date-parts": [
[
2025,
9,
1
]
],
"date-time": "2025-09-01T13:59:01Z",
"timestamp": 1756735141000
},
"funder": [
{
"DOI": "10.13039/501100001809",
"award": [
"82371428",
"82171533"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100001809",
"id-type": "DOI"
}
],
"name": "National Natural Science Foundation of China"
},
{
"DOI": "10.13039/501100003453",
"award": [
"2022A1515011623",
"2024A1515011035"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100003453",
"id-type": "DOI"
}
],
"name": "Natural Science Foundation of Guangdong Province"
},
{
"award": [
"202201003"
],
"name": "Science and Technology Program of Liwan District, Guangzhou"
},
{
"award": [
"2023A03J0852",
"2023A03J0853",
"2023A03J0850"
],
"name": "Guangzhou Science and Technology Plan Project—University-Enterprise Joint Funding"
},
{
"award": [
"2021ZD0201800"
],
"name": "National Brain Science and Brain-Like Intelligence Technology Project"
}
],
"indexed": {
"date-parts": [
[
2025,
9,
1
]
],
"date-time": "2025-09-01T14:40:01Z",
"timestamp": 1756737601461,
"version": "3.44.0"
},
"is-referenced-by-count": 0,
"issue": "9",
"issued": {
"date-parts": [
[
2025,
8,
31
]
]
},
"journal-issue": {
"issue": "9",
"published-online": {
"date-parts": [
[
2025,
9
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
8,
31
]
],
"date-time": "2025-08-31T00:00:00Z",
"timestamp": 1756598400000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2227-9059/13/9/2133/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "2133",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2025,
8,
31
]
]
},
"published-online": {
"date-parts": [
[
2025,
8,
31
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1001/jama.2022.10594",
"article-title": "Review of Antiseizure Medications for Adults With Epilepsy",
"author": "Majeed",
"doi-asserted-by": "crossref",
"first-page": "680",
"journal-title": "JAMA",
"key": "ref_1",
"volume": "328",
"year": "2022"
},
{
"DOI": "10.1038/s41573-024-00981-w",
"article-title": "New epilepsy therapies in development",
"author": "Klein",
"doi-asserted-by": "crossref",
"first-page": "682",
"journal-title": "Nat. Rev. Drug Discov.",
"key": "ref_2",
"volume": "23",
"year": "2024"
},
{
"DOI": "10.1038/s41582-019-0217-x",
"article-title": "Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy",
"author": "Vezzani",
"doi-asserted-by": "crossref",
"first-page": "459",
"journal-title": "Nat. Rev. Neurol.",
"key": "ref_3",
"volume": "15",
"year": "2019"
},
{
"DOI": "10.1016/j.scib.2025.02.036",
"article-title": "Nanoengineered, ultrasmall and catalytic potassium calcium hexacyanoferrate for neuroprotection and temporal lobe epilepsy treatment",
"author": "Feng",
"doi-asserted-by": "crossref",
"first-page": "1627",
"journal-title": "Sci. Bull.",
"key": "ref_4",
"volume": "70",
"year": "2025"
},
{
"DOI": "10.1002/ana.23567",
"article-title": "Seizure-induced brain-borne inflammation sustains seizure recurrence and blood-brain barrier damage",
"author": "Librizzi",
"doi-asserted-by": "crossref",
"first-page": "82",
"journal-title": "Ann. Neurol.",
"key": "ref_5",
"volume": "72",
"year": "2012"
},
{
"DOI": "10.1016/j.neuron.2024.03.017",
"article-title": "Microglial P2Y(6) calcium signaling promotes phagocytosis and shapes neuroimmune responses in epileptogenesis",
"author": "Umpierre",
"doi-asserted-by": "crossref",
"first-page": "1959",
"journal-title": "Neuron",
"key": "ref_6",
"volume": "112",
"year": "2024"
},
{
"DOI": "10.1016/j.redox.2024.103168",
"doi-asserted-by": "crossref",
"key": "ref_7",
"unstructured": "Liang, L.P., Sri Hari, A., Day, B.J., and Patel, M. (2024). Pharmacological elevation of glutathione inhibits status epilepticus-induced neuroinflammation and oxidative injury. Redox Biol., 73."
},
{
"DOI": "10.1016/j.nbd.2023.106027",
"doi-asserted-by": "crossref",
"key": "ref_8",
"unstructured": "Villasana-Salazar, B., and Vezzani, A. (2023). Neuroinflammation microenvironment sharpens seizure circuit. Neurobiol. Dis., 178."
},
{
"DOI": "10.1016/j.msard.2020.102535",
"article-title": "How does the COVID-19 cause seizure and epilepsy in patients? The potential mechanisms",
"author": "Nikbakht",
"doi-asserted-by": "crossref",
"first-page": "102535",
"journal-title": "Mult. Scler. Relat. Disord.",
"key": "ref_9",
"volume": "46",
"year": "2020"
},
{
"DOI": "10.1111/epi.16524",
"article-title": "New onset acute symptomatic seizure and risk factors in coronavirus disease 2019: A retrospective multicenter study",
"author": "Lu",
"doi-asserted-by": "crossref",
"first-page": "e49",
"journal-title": "Epilepsia",
"key": "ref_10",
"volume": "61",
"year": "2020"
},
{
"DOI": "10.1016/j.bbi.2020.06.008",
"article-title": "A brewing storm: The neuropsychological sequelae of hyperinflammation due to COVID-19",
"author": "Cothran",
"doi-asserted-by": "crossref",
"first-page": "957",
"journal-title": "Brain Behav. Immun.",
"key": "ref_11",
"volume": "88",
"year": "2020"
},
{
"DOI": "10.1186/s40035-024-00458-1",
"article-title": "SARS-CoV-2 membrane protein induces neurodegeneration via affecting Golgi-mitochondria interaction",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "68",
"journal-title": "Transl. Neurodegener.",
"key": "ref_12",
"volume": "13",
"year": "2024"
},
{
"DOI": "10.1136/jnnp-2020-324337",
"article-title": "Epileptiform activity and seizures in patients with COVID-19",
"author": "Issa",
"doi-asserted-by": "crossref",
"first-page": "565",
"journal-title": "J. Neurol. Neurosurg. Psychiatry",
"key": "ref_13",
"volume": "92",
"year": "2021"
},
{
"DOI": "10.1212/WNL.0000000000201651",
"article-title": "The Significance of the Increased Incidence of New Onset Seizures and Epilepsy After a COVID-19 Infection",
"author": "Bensken",
"doi-asserted-by": "crossref",
"first-page": "359",
"journal-title": "Neurology",
"key": "ref_14",
"volume": "100",
"year": "2023"
},
{
"DOI": "10.1212/WNL.0000000000200195",
"article-title": "Risk of COVID-19 Infection and of Severe Complications Among People With Epilepsy: A Nationwide Cohort Study",
"author": "Yoo",
"doi-asserted-by": "crossref",
"first-page": "e1886",
"journal-title": "Neurology",
"key": "ref_15",
"volume": "98",
"year": "2022"
},
{
"DOI": "10.3390/ijms25073819",
"doi-asserted-by": "crossref",
"key": "ref_16",
"unstructured": "Chagas, L.D.S., and Serfaty, C.A. (2024). The Influence of Microglia on Neuroplasticity and Long-Term Cognitive Sequelae in Long COVID: Impacts on Brain Development and Beyond. Int. J. Mol. Sci., 25."
},
{
"DOI": "10.1186/1756-6606-5-18",
"article-title": "Gabapentin reduces CX3CL1 signaling and blocks spinal microglial activation in monoarthritic rats",
"author": "Yang",
"doi-asserted-by": "crossref",
"first-page": "18",
"journal-title": "Mol. Brain",
"key": "ref_17",
"volume": "5",
"year": "2012"
},
{
"DOI": "10.1016/j.lfs.2023.121562",
"article-title": "Hepatoprotective potential of gabapentin in cecal ligation and puncture-induced sepsis; targeting oxidative stress, apoptosis, and NF-kB/MAPK signaling pathways",
"author": "Abdelnaser",
"doi-asserted-by": "crossref",
"first-page": "121562",
"journal-title": "Life Sci.",
"key": "ref_18",
"volume": "320",
"year": "2023"
},
{
"DOI": "10.3390/ph10040093",
"doi-asserted-by": "crossref",
"key": "ref_19",
"unstructured": "Rossi, A., Murta, V., Auzmendi, J., and Ramos, A.J. (2017). Early Gabapentin Treatment during the Latency Period Increases Convulsive Threshold, Reduces Microglial Activation and Macrophage Infiltration in the Lithium-Pilocarpine Model of Epilepsy. Pharm., 10."
},
{
"DOI": "10.3389/fphar.2025.1500475",
"doi-asserted-by": "crossref",
"key": "ref_20",
"unstructured": "Deng, N.J., Li, X.Y., Zhang, Z.X., Xian-Yu, C.Y., Tao, Y.T., Ma, Y.T., Li, H.J., Gao, T.Y., Liu, X., and Luo, J. (2025). Effectiveness and safety of single anti-seizure medication as adjunctive therapy for drug-resistant focal epilepsy based on network meta-analysis. Front. Pharmacol., 16."
},
{
"DOI": "10.3346/jkms.2013.28.2.308",
"article-title": "Intrathecal gabapentin increases interleukin-10 expression and inhibits pro-inflammatory cytokine in a rat model of neuropathic pain",
"author": "Lee",
"doi-asserted-by": "crossref",
"first-page": "308",
"journal-title": "J. Korean Med. Sci.",
"key": "ref_21",
"volume": "28",
"year": "2013"
},
{
"DOI": "10.1136/bcr-2021-243459",
"article-title": "Neuropathic pain post-COVID-19: A case report",
"author": "McWilliam",
"doi-asserted-by": "crossref",
"first-page": "e243459",
"journal-title": "BMJ Case Rep.",
"key": "ref_22",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.1111/crj.13529",
"article-title": "The effectiveness of gabapentin and gabapentin/montelukast combination compared with dextromethorphan in the improvement of COVID-19- related cough: A randomized, controlled clinical trial",
"author": "Soltani",
"doi-asserted-by": "crossref",
"first-page": "604",
"journal-title": "Clin. Respir. J.",
"key": "ref_23",
"volume": "16",
"year": "2022"
},
{
"DOI": "10.1002/wjo2.176",
"article-title": "Clinical studies in COVID-related olfactory disorders: Review of an institutional experience",
"author": "Tharakan",
"doi-asserted-by": "crossref",
"first-page": "129",
"journal-title": "World J. Otorhinolaryngol. Head. Neck Surg.",
"key": "ref_24",
"volume": "10",
"year": "2024"
},
{
"DOI": "10.1002/alr.23117",
"article-title": "Gabapentin improves parosmia after COVID-19 infection",
"author": "Garcia",
"doi-asserted-by": "crossref",
"first-page": "1034",
"journal-title": "Int. Forum Allergy Rhinol.",
"key": "ref_25",
"volume": "13",
"year": "2023"
},
{
"article-title": "[Vertical transmission of SARS-CoV-2. Impact on the nervous system]",
"first-page": "S215",
"journal-title": "Rev. Med. Inst. Mex. Seguro Soc.",
"key": "ref_26",
"volume": "58",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(21)00095-7",
"article-title": "Neuropathology associated with SARS-CoV-2 infection",
"author": "Egervari",
"doi-asserted-by": "crossref",
"first-page": "276",
"journal-title": "Lancet",
"key": "ref_27",
"volume": "397",
"year": "2021"
},
{
"article-title": "High rate of epilepsy in young individuals who died with COVID-19",
"author": "Lempriere",
"first-page": "65",
"journal-title": "Nat. Rev. Neurol.",
"key": "ref_28",
"volume": "18",
"year": "2022"
},
{
"DOI": "10.1212/WNL.0000000000200367",
"article-title": "Epilepsy and COVID-19’s Double-Edged Sword: More Severe Disease and Delayed Epilepsy Care",
"author": "Berg",
"doi-asserted-by": "crossref",
"first-page": "779",
"journal-title": "Neurology",
"key": "ref_29",
"volume": "98",
"year": "2022"
},
{
"DOI": "10.3390/antiox12051020",
"doi-asserted-by": "crossref",
"key": "ref_30",
"unstructured": "Kim, J.E., Lee, D.S., Kim, T.H., Park, H., and Kang, T.C. (2023). Distinct Roles of CK2- and AKT-Mediated NF-kappaB Phosphorylations in Clasmatodendrosis (Autophagic Astroglial Death) within the Hippocampus of Chronic Epilepsy Rats. Antioxidants, 12."
},
{
"DOI": "10.1016/j.phrs.2021.105877",
"article-title": "TLR4 and AT1R mediate blood-brain barrier disruption, neuroinflammation, and autonomic dysfunction in spontaneously hypertensive rats",
"author": "Mowry",
"doi-asserted-by": "crossref",
"first-page": "105877",
"journal-title": "Pharmacol. Res.",
"key": "ref_31",
"volume": "174",
"year": "2021"
},
{
"DOI": "10.1161/CIRCULATIONAHA.108.814582",
"article-title": "Myeloid-related protein-8/14 is critical for the biological response to vascular injury",
"author": "Croce",
"doi-asserted-by": "crossref",
"first-page": "427",
"journal-title": "Circulation",
"key": "ref_32",
"volume": "120",
"year": "2009"
},
{
"DOI": "10.1016/j.bbi.2019.08.195",
"article-title": "TNF deficiency causes alterations in the spatial organization of neurogenic zones and alters the number of microglia and neurons in the cerebral cortex",
"author": "Larsen",
"doi-asserted-by": "crossref",
"first-page": "279",
"journal-title": "Brain Behav. Immun.",
"key": "ref_33",
"volume": "82",
"year": "2019"
},
{
"DOI": "10.1016/j.bbi.2024.05.023",
"article-title": "Opposing effects of the purinergic P2X7 receptor on seizures in neurons and microglia in male mice",
"author": "Alves",
"doi-asserted-by": "crossref",
"first-page": "121",
"journal-title": "Brain Behav. Immun.",
"key": "ref_34",
"volume": "120",
"year": "2024"
},
{
"DOI": "10.3389/fimmu.2019.00121",
"doi-asserted-by": "crossref",
"key": "ref_35",
"unstructured": "Owens, G.C., Garcia, A.J., Mochizuki, A.Y., Chang, J.W., Reyes, S.D., Salamon, N., Prins, R.M., Mathern, G.W., and Fallah, A. (2019). Evidence for Innate and Adaptive Immune Responses in a Cohort of Intractable Pediatric Epilepsy Surgery Patients. Front. Immunol., 10."
},
{
"DOI": "10.1161/ATVBAHA.122.318721",
"article-title": "Monocyte-Mediated Thrombosis Linked to Circulating Tissue Factor and Immune Paralysis in COVID-19",
"author": "Goonewardena",
"doi-asserted-by": "crossref",
"first-page": "1124",
"journal-title": "Arterioscler. Thromb. Vasc. Biol.",
"key": "ref_36",
"volume": "44",
"year": "2024"
},
{
"DOI": "10.1016/j.bbi.2020.12.026",
"article-title": "Platelet endothelial cell adhesion molecule-1 is a gatekeeper of neutrophil transendothelial migration in ischemic stroke",
"author": "Winneberger",
"doi-asserted-by": "crossref",
"first-page": "277",
"journal-title": "Brain Behav. Immun.",
"key": "ref_37",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1038/s41590-020-00828-7",
"article-title": "Proinflammatory IgG Fc structures in patients with severe COVID-19",
"author": "Chakraborty",
"doi-asserted-by": "crossref",
"first-page": "67",
"journal-title": "Nat. Immunol.",
"key": "ref_38",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1016/j.nbd.2014.11.009",
"article-title": "Role of fractalkine-CX3CR1 pathway in seizure-induced microglial activation, neurodegeneration, and neuroblast production in the adult rat brain",
"author": "Ali",
"doi-asserted-by": "crossref",
"first-page": "194",
"journal-title": "Neurobiol. Dis.",
"key": "ref_39",
"volume": "74",
"year": "2015"
},
{
"DOI": "10.1016/j.jneuroim.2011.03.005",
"article-title": "The roles of fractalkine/CX3CR1 system in neuronal death following pilocarpine-induced status epilepticus",
"author": "Yeo",
"doi-asserted-by": "crossref",
"first-page": "93",
"journal-title": "J. Neuroimmunol.",
"key": "ref_40",
"volume": "234",
"year": "2011"
},
{
"DOI": "10.1111/imcb.12464",
"article-title": "Monocytes and macrophages in severe COVID-19—Friend, foe or both?",
"author": "Dress",
"doi-asserted-by": "crossref",
"first-page": "561",
"journal-title": "Immunol. Cell Biol.",
"key": "ref_41",
"volume": "99",
"year": "2021"
},
{
"DOI": "10.2147/DDDT.S314076",
"article-title": "Baicalin Rescues Cognitive Dysfunction, Mitigates Neurodegeneration, and Exerts Anti-Epileptic Effects Through Activating TLR4/MYD88/Caspase-3 Pathway in Rats",
"author": "Yang",
"doi-asserted-by": "crossref",
"first-page": "3163",
"journal-title": "Drug Des. Devel Ther.",
"key": "ref_42",
"volume": "15",
"year": "2021"
},
{
"DOI": "10.1186/s13578-020-00387-2",
"doi-asserted-by": "crossref",
"key": "ref_43",
"unstructured": "Liu, L., Xu, Y., Dai, H., Tan, S., Mao, X., and Chen, Z. (2020). Dynorphin activation of kappa opioid receptor promotes microglial polarization toward M2 phenotype via TLR4/NF-kappaB pathway. Cell Biosci., 10."
},
{
"DOI": "10.1080/14728222.2021.1918103",
"article-title": "TLR4 as a therapeutic target for respiratory and neurological complications of SARS-CoV-2",
"author": "Kaushik",
"doi-asserted-by": "crossref",
"first-page": "491",
"journal-title": "Expert. Opin. Ther. Targets",
"key": "ref_44",
"volume": "25",
"year": "2021"
},
{
"DOI": "10.20944/preprints202309.2036.v2",
"doi-asserted-by": "crossref",
"key": "ref_45",
"unstructured": "Kyriatzis, G., Bernard, A., Bole, A., Khrestchatisky, M., and Ferhat, L. (2024). In the Rat Hippocampus, Pilocarpine-Induced Status Epilepticus Is Associated with Reactive Glia and Concomitant Increased Expression of CD31, PDGFRbeta, and Collagen IV in Endothelial Cells and Pericytes of the Blood-Brain Barrier. Int. J. Mol. Sci., 25."
},
{
"DOI": "10.1016/j.ajt.2023.12.005",
"article-title": "Natural killer cell functional genetics and donor-specific antibody-triggered microvascular inflammation",
"author": "Diebold",
"doi-asserted-by": "crossref",
"first-page": "743",
"journal-title": "Am. J. Transplant.",
"key": "ref_46",
"volume": "24",
"year": "2024"
},
{
"DOI": "10.1002/eji.201646837",
"article-title": "CD69: From activation marker to metabolic gatekeeper",
"author": "Cibrian",
"doi-asserted-by": "crossref",
"first-page": "946",
"journal-title": "Eur. J. Immunol.",
"key": "ref_47",
"volume": "47",
"year": "2017"
},
{
"DOI": "10.1016/j.brainres.2015.07.034",
"article-title": "Epigenetics and therapeutic targets mediating neuroprotection",
"author": "Qureshi",
"doi-asserted-by": "crossref",
"first-page": "265",
"journal-title": "Brain Res.",
"key": "ref_48",
"volume": "1628",
"year": "2015"
},
{
"DOI": "10.1016/j.jhep.2015.08.033",
"article-title": "NF-kappaB/RelA and Nrf2 cooperate to maintain hepatocyte integrity and to prevent development of hepatocellular adenoma",
"author": "Kohler",
"doi-asserted-by": "crossref",
"first-page": "94",
"journal-title": "J. Hepatol.",
"key": "ref_49",
"volume": "64",
"year": "2016"
},
{
"DOI": "10.1007/s11481-023-10091-w",
"article-title": "beta-Sitosterol Alleviates Neuropathic Pain by Affect Microglia Polarization through Inhibiting TLR4/NF-kappaB Signaling Pathway",
"author": "Zheng",
"doi-asserted-by": "crossref",
"first-page": "690",
"journal-title": "J. Neuroimmune Pharmacol.",
"key": "ref_50",
"volume": "18",
"year": "2023"
},
{
"DOI": "10.1016/j.bbi.2021.04.007",
"article-title": "Microglial IL-10 and beta-endorphin expression mediates gabapentinoids antineuropathic pain",
"author": "Ahmad",
"doi-asserted-by": "crossref",
"first-page": "344",
"journal-title": "Brain Behav. Immun.",
"key": "ref_51",
"volume": "95",
"year": "2021"
},
{
"DOI": "10.1016/j.pharmthera.2021.107989",
"article-title": "CX3CL1/CX3CR1 signaling targets for the treatment of neurodegenerative diseases",
"author": "Subbarayan",
"doi-asserted-by": "crossref",
"first-page": "107989",
"journal-title": "Pharmacol. Ther.",
"key": "ref_52",
"volume": "231",
"year": "2022"
},
{
"DOI": "10.1001/jama.2022.13659",
"article-title": "Growing Role of Gabapentin in Opioid-Related Overdoses Highlights Misuse Potential and Off-label Prescribing Practices",
"author": "Kuehn",
"doi-asserted-by": "crossref",
"first-page": "1283",
"journal-title": "JAMA",
"key": "ref_53",
"volume": "328",
"year": "2022"
},
{
"DOI": "10.3390/medicines10090052",
"doi-asserted-by": "crossref",
"key": "ref_54",
"unstructured": "Rissardo, J.P., Medeiros Araujo de Matos, U., and Fornari Caprara, A.L. (2023). Gabapentin-Associated Movement Disorders: A Literature Review. Medicines, 10."
},
{
"DOI": "10.5146/tjpath.2025.14236",
"doi-asserted-by": "crossref",
"key": "ref_55",
"unstructured": "El-Kattan, M.A., Saeed, E., Khattab, M.A., Maksoud, F., Eldein, M.E., Abdel-Roaf, N.E., Awad, W., and Elshatory, A. (2025). Gabapentin-Induced Sub-Chronic Neurotoxicity in Rats and the Protective Role of Alpha-Tocopherol. Turk. Patoloji Derg., Online ahead of print."
}
],
"reference-count": 55,
"references-count": 55,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2227-9059/13/9/2133"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "CX3CR1–TLR4 Axis as a Shared Neuroimmune Target in COVID-19 and Epilepsy: Integrative Transcriptomics and Gabapentin Repositioning",
"type": "journal-article",
"volume": "13"
}