Clinical Significance of Micronutrient Supplementation in Critically Ill COVID-19 Patients with Severe ARDS
et al., Nutrients, doi:10.3390/nu13062113, Jun 2021
Zinc for COVID-19
2nd treatment shown to reduce risk in
July 2020, now with p = 0.00000028 from 47 studies, recognized in 23 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
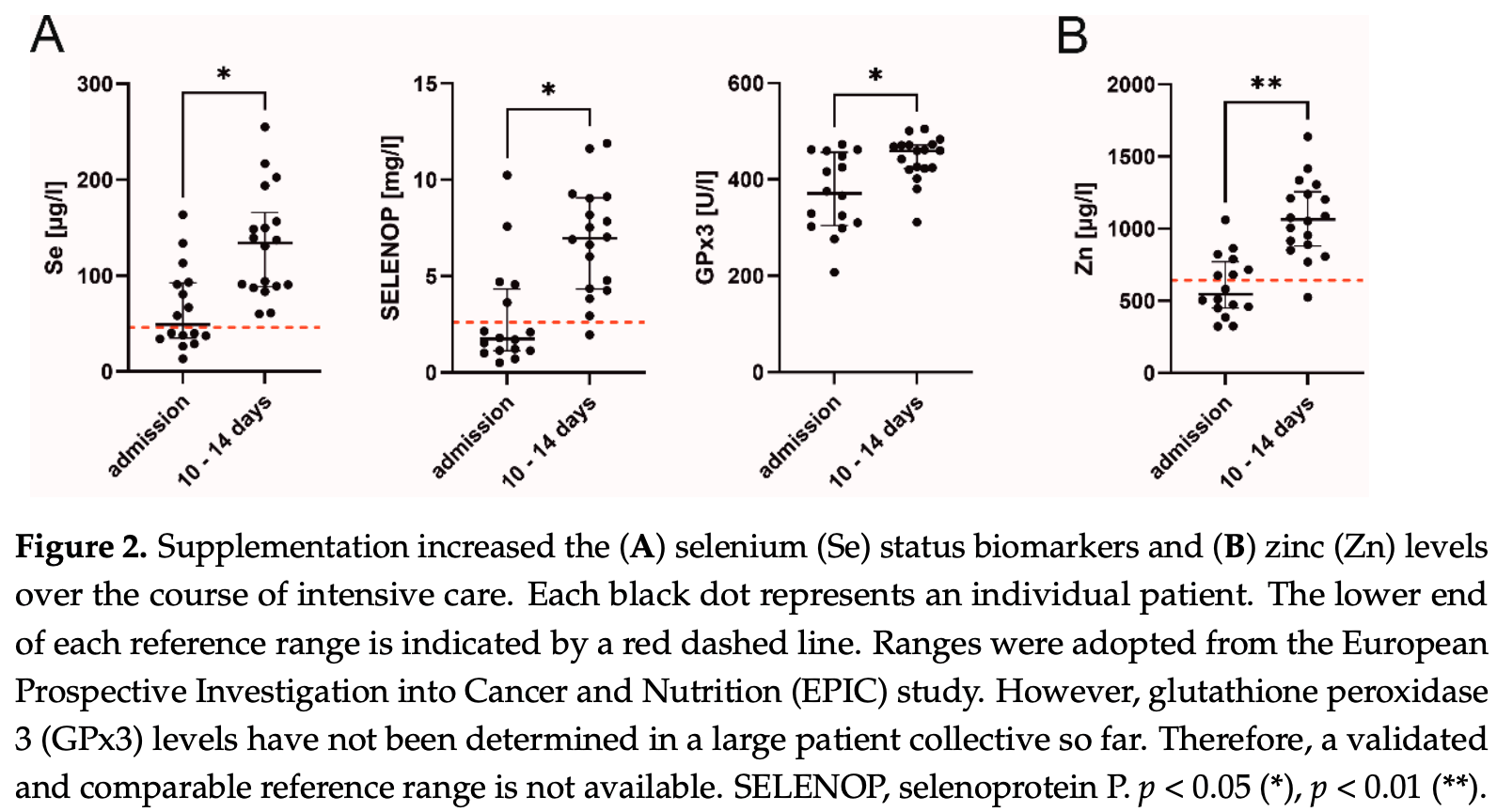
Retrospective 22 ICU patients, showing most patients had low selenium status biomarkers and low zinc levels, and reporting on treatment with nutrient supplementation including selenium and zinc. Authors conclude that sufficient selenium and zinc levels may be important for an adequate immune response in critically ill patients with severe COVID-19 ARDS.
Study covers selenium and zinc.
Notz et al., 20 Jun 2021, retrospective, Germany, peer-reviewed, 13 authors.
Clinical Significance of Micronutrient Supplementation in Critically Ill COVID-19 Patients with Severe ARDS
Nutrients, doi:10.3390/nu13062113
The interplay between inflammation and oxidative stress is a vicious circle, potentially resulting in organ damage. Essential micronutrients such as selenium (Se) and zinc (Zn) support anti-oxidative defense systems and are commonly depleted in severe disease. This single-center retrospective study investigated micronutrient levels under Se and Zn supplementation in critically ill patients with COVID-19 induced acute respiratory distress syndrome (ARDS) and explored potential relationships with immunological and clinical parameters. According to intensive care unit (ICU) standard operating procedures, patients received 1.0 mg of intravenous Se daily on top of artificial nutrition, which contained various amounts of Se and Zn. Micronutrients, inflammatory cytokines, lymphocyte subsets and clinical data were extracted from the patient data management system on admission and after 10 to 14 days of treatment. Forty-six patients were screened for eligibility and 22 patients were included in the study. Twenty-one patients (95%) suffered from severe ARDS and 14 patients (64%) survived to ICU discharge. On admission, the majority of patients had low Se status biomarkers and Zn levels, along with elevated inflammatory parameters. Se supplementation significantly elevated Se (p = 0.027) and selenoprotein P levels (SELENOP; p = 0.016) to normal range. Accordingly, glutathione peroxidase 3 (GPx3) activity increased over time (p = 0.021). Se biomarkers, most notably SELENOP, were inversely correlated with CRP (r s = −0.495), PCT (r s = −0.413), IL-6 (r s = −0.429), IL-1β (r s = −0.440) and IL-10 (r s = −0.461). Positive associations were found for CD8 + T cells (r s = 0.636), NK cells (r s = 0.772), total IgG (r s = 0.493) and PaO 2 /FiO 2 ratios (r s = 0.504). In addition, survivors tended to have higher Se levels after 10 to 14 days compared to non-survivors (p = 0.075). Sufficient Se and Zn levels may potentially be of clinical significance for an adequate immune response in critically ill patients with severe COVID-19 ARDS.
Conflicts of Interest: Stephan Sudowe is employed at Ganzimmun Diagnostics AG. Lutz Schomburg holds shares in selenOmed GmbH, a company involved in Se status assessment and supplementation. Christian Stoppe reports grants and non-financial support from Biosyn Arzneimittel GmbH, outside the submitted work. The other authors declare no competing interest.
References
Allingstrup, Afshari, Selenium supplementation for critically ill adults, Cochrane Database Syst. Rev, doi:10.1002/14651858.CD003703.pub3
Angstwurm, Engelmann, Zimmermann, Lehmann, Spes et al., Selenium in Intensive Care (SIC): Results of a prospective randomized, placebo-controlled, multiple-center study in patients with severe systemic inflammatory response syndrome, sepsis, and septic shock, Crit. Care Med, doi:10.1097/01.CCM.0000251124.83436.0E
Arentz, Yim, Klaff, Lokhandwala, Riedo et al., Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State, JAMA, doi:10.1001/jama.2020.4326
Avery, Hoffmann, Selenium, Selenoproteins, and Immunity, Nutrients, doi:10.3390/nu10091203
Bhatraju, Ghassemieh, Nichols, Kim, Jerome et al., Covid-19 in Critically Ill Patients in the Seattle Region-Case Series, N. Engl. J. Med, doi:10.1056/NEJMoa2004500
Coppolino, Leonardi, Andreucci, Bolignano, Oxidative Stress and Kidney Function: A Brief Update, Curr. Pharm. Des, doi:10.2174/1381612825666190112165206
Corman, Landt, Kaiser, Molenkamp, Meijer et al., Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR, Euro Surveill, doi:10.2807/1560-7917.ES.2020.25.3.2000045
Flohé, Günzler, Assays of glutathione peroxidase, Methods Enzym, doi:10.1016/s0076-6879(84)05015-1
Forceville, Laviolle, Annane, Vitoux, Bleichner et al., Effects of high doses of selenium, as sodium selenite, in septic shock: A placebo-controlled, randomized, double-blind, phase II study, Crit. Care, doi:10.1186/cc5960
Forceville, Vitoux, Gauzit, Combes, Lahilaire et al., Selenium, systemic immune response syndrome, sepsis, and outcome in critically ill patients, Crit. Care Med, doi:10.1097/00003246-199809000-00021
Gombart, Pierre, Maggini, A Review of Micronutrients and the Immune System-Working in Harmony to Reduce the Risk of Infection, Nutrients, doi:10.3390/nu12010236
Grasselli, Greco, Zanella, Albano, Antonelli et al., Risk Factors Associated with Mortality Among Patients With COVID-19 in Intensive Care Units in Lombardy, Italy, JAMA Intern. Med, doi:10.1001/jamainternmed.2020.3539
Guillin, Vindry, Ohlmann, Chavatte, Selenium, Selenoproteins and Viral Infection, Nutrients, doi:10.3390/nu11092101
Heller, Sun, Hackler, Seelig, Seibert et al., Prediction of survival odds in COVID-19 by zinc, age and selenoprotein P as composite biomarker, Redox Biol, doi:10.1016/j.redox.2020.101764
Heyland, Muscedere, Wischmeyer, Cook, Jones et al., A randomized trial of glutamine and antioxidants in critically ill patients, N. Engl. J. Med, doi:10.1056/NEJMoa1212722
Huang, Shyu, Chen, Lin, Lo et al., Effect of parenteral selenium supplementation in critically ill patients: A systematic review and meta-analysis, PLoS ONE, doi:10.1371/journal.pone.0054431
Huang, Wang, Li, Ren, Zhao et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet, doi:10.1016/S0140-6736(20)30183-5
Hughes, Fedirko, Jenab, Schomburg, Méplan et al., Selenium status is associated with colorectal cancer risk in the European prospective investigation of cancer and nutrition cohort, Int. J. Cancer, doi:10.1002/ijc.29071
Hui, Azhar, Madani, Ntoumi, Kock et al., The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health-The latest 2019 novel coronavirus outbreak in Wuhan, China, Int. J. Infect. Dis, doi:10.1016/j.ijid.2020.01.009
Imai, Kuba, Neely, Yaghubian-Malhami, Perkmann et al., Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury, Cell, doi:10.1016/j.cell.2008.02.043
Jo, Kim, Shin, Sasakawa, Molecular mechanisms regulating NLRP3 inflammasome activation, Cell Mol. Immunol, doi:10.1038/cmi.2015.95
Leisman, Ronner, Pinotti, Taylor, Sinha et al., Cytokine elevation in severe and critical COVID-19: A rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes, Lancet Respir. Med, doi:10.1016/S2213-2600(20)30404-5
Li, Zhao, Zhang, Sun, Jia et al., Se deficiency induces renal pathological changes by regulating selenoprotein expression, disrupting redox balance, and activating inflammation, Metallomics, doi:10.1039/D0MT00165A
Liu, Li, Xu, Wu, Luo et al., Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19, J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol, doi:10.1016/j.jcv.2020.104370
Lopez-Castejon, Brough, Understanding the mechanism of IL-1β secretion, Cytokine Growth Factor Rev, doi:10.1016/j.cytogfr.2011.10.001
Luo, Zhou, Yan, Guo, Wang et al., Prognostic Value of C-Reactive Protein in Patients With Coronavirus, Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am, doi:10.1093/cid/ciaa641
Maares, Haase, Zinc and immunity: An essential interrelation, Arch. Biochem. Biophys, doi:10.1016/j.abb.2016.03.022
Maeda, Baba, Nagai, Miyazaki, Malykhin et al., IL-6 blocks a discrete early step in lymphopoiesis, Blood, doi:10.1182/blood-2005-02-0456
Mahmoodpoor, Hamishehkar, Shadvar, Ostadi, Sanaie et al., The Effect of Intravenous Selenium on Oxidative Stress in Critically Ill Patients with Acute Respiratory Distress Syndrome, Immunol. Investig, doi:10.1080/08820139.2018.1496098
Manzanares, Biestro, Galusso, Torre, Mañay et al., Serum selenium and glutathione peroxidase-3 activity: Biomarkers of systemic inflammation in the critically ill?, Intensive Care Med, doi:10.1007/s00134-008-1356-5
Manzanares, Lemieux, Elke, Langlois, Bloos et al., High-dose intravenous selenium does not improve clinical outcomes in the critically ill: A systematic review and meta-analysis, Crit. Care, doi:10.1186/s13054-016-1529-5
Manzanares, Moreira, Hardy, Pharmaconutrition revisited for critically ill patients with coronavirus disease 2019 (COVID-19): Does selenium have a place?, Nutrition, doi:10.1016/j.nut.2020.110989
Martitz, Becker, Renko, Stoedter, Hybsier et al., Gene-specific regulation of hepatic selenoprotein expression by interleukin-6, Metallomics, doi:10.1039/C5MT00211G
Meduri, Annane, Chrousos, Marik, Sinclair, Activation and regulation of systemic inflammation in ARDS: Rationale for prolonged glucocorticoid therapy, Chest, doi:10.1378/chest.08-2408
Mehta, Mcauley, Brown, Sanchez, Tattersall et al., COVID-19: Consider cytokine storm syndromes and immunosuppression, Lancet, doi:10.1016/S0140-6736(20)30628-0
Moghaddam, Heller, Sun, Seelig, Cherkezov et al., Selenium Deficiency Is Associated with Mortality Risk from COVID-19, Nutrients, doi:10.3390/nu12072098
Morbach, Eichhorn, Liese, Girschick, Reference values for B cell subpopulations from infancy to adulthood, Clin. Exp. Immunol, doi:10.1111/j.1365-2249.2010.04206.x
Motoyama, Okamoto, Kukita, Hamaguchi, Kinoshita et al., Possible role of increased oxidant stress in multiple organ failure after systemic inflammatory response syndrome, Crit. Care Med, doi:10.1097/01.CCM.0000055371.27268.36
Mrityunjaya, Pavithra, Neelam, Janhavi, Halami et al., Immune-Boosting, Antioxidant and Antiinflammatory Food Supplements Targeting Pathogenesis of COVID-19, Front. Immunol, doi:10.3389/fimmu.2020.570122
Notz, Schmalzing, Wedekink, Schlesinger, Gernert et al., Pro-and Anti-Inflammatory Responses in Severe COVID-19-Induced Acute Respiratory Distress Syndrome-An Observational Pilot Study, Front. Immunol, doi:10.3389/fimmu.2020.581338
Prauchner, Oxidative stress in sepsis: Pathophysiological implications justifying antioxidant co-therapy, Burns, doi:10.1016/j.burns.2016.09.023
Qin, Zhou, Hu, Zhang, Yang et al., Dysregulation of immune response in patients with COVID-19 in Wuhan, China, Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am, doi:10.2139/ssrn.3541136
Ranieri, Rubenfeld, Thompson, Ferguson, Caldwell et al., Acute respiratory distress syndrome: The Berlin Definition, JAMA, doi:10.1001/jama.2012.5669
Saleh, Peyssonnaux, Singh, Edeas, Mitochondria and microbiota dysfunction in COVID-19 pathogenesis, Mitochondrion, doi:10.1016/j.mito.2020.06.008
Schlesinger, Weißbrich, Wedekink, Notz, Herrmann et al., Biodistribution and serologic response in SARS-CoV-2 induced ARDS: A cohort study, PLoS ONE, doi:10.1371/journal.pone.0242917
Schmidt, Pargger, Seeberger, Eckhart, Von Felten et al., Effect of high-dose sodium selenite in cardiac surgery patients: A randomized controlled bi-center trial, Clin. Nutr. Edinb. Scotl, doi:10.1016/j.clnu.2017.04.019
Shankar, Prasad, Zinc and immune function: The biological basis of altered resistance to infection, Am. J. Clin. Nutr, doi:10.1093/ajcn/68.2.447S
Singer, Blaser, Berger, Alhazzani, Calder et al., ESPEN guideline on clinical nutrition in the intensive care unit, Clin. Nutr. Edinb. Scotl, doi:10.1016/j.clnu.2018.08.037
Singh, Kukreti, Saso, Kukreti, Oxidative Stress: A Key Modulator in Neurodegenerative Diseases, Molecules, doi:10.3390/molecules24081583
Steinbrenner, Al-Quraishy, Dkhil, Wunderlich, Sies, Dietary selenium in adjuvant therapy of viral and bacterial infections, Adv. Nutr. Bethesda Md, doi:10.3945/an.114.007575
Stepien, Jenab, Freisling, Becker, Czuban et al., Pre-diagnostic copper and zinc biomarkers and colorectal cancer risk in the European Prospective Investigation into Cancer and Nutrition cohort, Carcinogenesis, doi:10.1093/carcin/bgx051
Stoppe, Spillner, Rossaint, Coburn, Schälte et al., Selenium blood concentrations in patients undergoing elective cardiac surgery and receiving perioperative sodium selenite, Nutrition, doi:10.1016/j.nut.2012.05.013
Takebe, Yarimizu, Saito, Hayashi, Nakamura et al., A comparative study on the hydroperoxide and thiol specificity of the glutathione peroxidase family and selenoprotein P, J. Biol. Chem
Vabret, Britton, Gruber, Hegde, Kim et al., Immunology of COVID-19: Current State of the Science, Immunity, doi:10.1016/j.immuni.2020.05.002
Vandenbroucke, Von Elm, Altman, Gøtzsche, Mulrow et al., Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration, Ann. Intern. Med, doi:10.7326/0003-4819-147-8-200710160-00010-w1
Wang, Tan, Li, Xu, Guo et al., Supplementation of Micronutrient Selenium in Metabolic Diseases: Its Role as an Antioxidant, Oxid. Med. Cell. Longev, doi:10.1155/2017/7478523
Warnatz, Schlesier, Flowcytometric phenotyping of common variable immunodeficiency, Cytometry. Part B Clin. Cytom, doi:10.1002/cyto.b.20432
Wessels, Haase, Engelhardt, Rink, Uciechowski, Zinc deficiency induces production of the proinflammatory cytokines IL-1β and TNFα in promyeloid cells via epigenetic and redox-dependent mechanisms, J. Nutr. Biochem, doi:10.1016/j.jnutbio.2012.06.007
Wessels, Maywald, Rink, Zinc as a Gatekeeper of Immune Function, Nutrients, doi:10.3390/nu9121286
Wessels, Rolles, Rink, The Potential Impact of Zinc Supplementation on COVID-19 Pathogenesis, Front. Immunol, doi:10.3389/fimmu.2020.01712
Wu, Leung, Leung, Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: A modelling study, Lancet, doi:10.1016/S0140-6736(20)30260-9
Xiang, Feng, Diao, Tu, Qiao et al., SARS-CoV-2 Induces Lymphocytopenia by Promoting Inflammation and Decimates Secondary Lymphoid Organs, Front. Immunol
Yang, Yu, Xu, Shu, Xia et al., Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study, Lancet Respir. Med, doi:10.1016/S2213-2600(20)30079-5
Zheng, Gao, Wang, Song, Liu et al., Functional exhaustion of antiviral lymphocytes in COVID-19 patients, Cell. Mol. Immunol, doi:10.1038/s41423-020-0402-2
DOI record:
{
"DOI": "10.3390/nu13062113",
"ISSN": [
"2072-6643"
],
"URL": "http://dx.doi.org/10.3390/nu13062113",
"abstract": "<jats:p>The interplay between inflammation and oxidative stress is a vicious circle, potentially resulting in organ damage. Essential micronutrients such as selenium (Se) and zinc (Zn) support anti-oxidative defense systems and are commonly depleted in severe disease. This single-center retrospective study investigated micronutrient levels under Se and Zn supplementation in critically ill patients with COVID-19 induced acute respiratory distress syndrome (ARDS) and explored potential relationships with immunological and clinical parameters. According to intensive care unit (ICU) standard operating procedures, patients received 1.0 mg of intravenous Se daily on top of artificial nutrition, which contained various amounts of Se and Zn. Micronutrients, inflammatory cytokines, lymphocyte subsets and clinical data were extracted from the patient data management system on admission and after 10 to 14 days of treatment. Forty-six patients were screened for eligibility and 22 patients were included in the study. Twenty-one patients (95%) suffered from severe ARDS and 14 patients (64%) survived to ICU discharge. On admission, the majority of patients had low Se status biomarkers and Zn levels, along with elevated inflammatory parameters. Se supplementation significantly elevated Se (p = 0.027) and selenoprotein P levels (SELENOP; p = 0.016) to normal range. Accordingly, glutathione peroxidase 3 (GPx3) activity increased over time (p = 0.021). Se biomarkers, most notably SELENOP, were inversely correlated with CRP (rs = −0.495), PCT (rs = −0.413), IL-6 (rs = −0.429), IL-1β (rs = −0.440) and IL-10 (rs = −0.461). Positive associations were found for CD8+ T cells (rs = 0.636), NK cells (rs = 0.772), total IgG (rs = 0.493) and PaO2/FiO2 ratios (rs = 0.504). In addition, survivors tended to have higher Se levels after 10 to 14 days compared to non-survivors (p = 0.075). Sufficient Se and Zn levels may potentially be of clinical significance for an adequate immune response in critically ill patients with severe COVID-19 ARDS.</jats:p>",
"alternative-id": [
"nu13062113"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-4042-4436",
"affiliation": [],
"authenticated-orcid": false,
"family": "Notz",
"given": "Quirin",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-2169-3761",
"affiliation": [],
"authenticated-orcid": false,
"family": "Herrmann",
"given": "Johannes",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schlesinger",
"given": "Tobias",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Helmer",
"given": "Philipp",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sudowe",
"given": "Stephan",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4458-6555",
"affiliation": [],
"authenticated-orcid": false,
"family": "Sun",
"given": "Qian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hackler",
"given": "Julian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Roeder",
"given": "Daniel",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2574-624X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Lotz",
"given": "Christopher",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2666-8696",
"affiliation": [],
"authenticated-orcid": false,
"family": "Meybohm",
"given": "Patrick",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5324-981X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kranke",
"given": "Peter",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9445-1555",
"affiliation": [],
"authenticated-orcid": false,
"family": "Schomburg",
"given": "Lutz",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2028-2039",
"affiliation": [],
"authenticated-orcid": false,
"family": "Stoppe",
"given": "Christian",
"sequence": "additional"
}
],
"container-title": "Nutrients",
"container-title-short": "Nutrients",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
6,
21
]
],
"date-time": "2021-06-21T01:50:15Z",
"timestamp": 1624240215000
},
"deposited": {
"date-parts": [
[
2021,
6,
21
]
],
"date-time": "2021-06-21T01:56:54Z",
"timestamp": 1624240614000
},
"indexed": {
"date-parts": [
[
2024,
5,
9
]
],
"date-time": "2024-05-09T16:36:35Z",
"timestamp": 1715272595114
},
"is-referenced-by-count": 36,
"issue": "6",
"issued": {
"date-parts": [
[
2021,
6,
20
]
]
},
"journal-issue": {
"issue": "6",
"published-online": {
"date-parts": [
[
2021,
6
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
6,
20
]
],
"date-time": "2021-06-20T00:00:00Z",
"timestamp": 1624147200000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2072-6643/13/6/2113/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "2113",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2021,
6,
20
]
]
},
"published-online": {
"date-parts": [
[
2021,
6,
20
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1016/S0140-6736(20)30260-9",
"doi-asserted-by": "publisher",
"key": "ref1"
},
{
"DOI": "10.1016/j.ijid.2020.01.009",
"doi-asserted-by": "publisher",
"key": "ref2"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"doi-asserted-by": "publisher",
"key": "ref3"
},
{
"DOI": "10.2139/ssrn.3541136",
"doi-asserted-by": "publisher",
"key": "ref4"
},
{
"DOI": "10.1016/j.mito.2020.06.008",
"doi-asserted-by": "publisher",
"key": "ref5"
},
{
"DOI": "10.1038/cmi.2015.95",
"doi-asserted-by": "publisher",
"key": "ref6"
},
{
"DOI": "10.1016/S0140-6736(20)30628-0",
"doi-asserted-by": "publisher",
"key": "ref7"
},
{
"DOI": "10.3390/molecules24081583",
"doi-asserted-by": "publisher",
"key": "ref8"
},
{
"DOI": "10.2174/1381612825666190112165206",
"doi-asserted-by": "publisher",
"key": "ref9"
},
{
"DOI": "10.1016/j.cell.2008.02.043",
"doi-asserted-by": "publisher",
"key": "ref10"
},
{
"DOI": "10.1016/j.burns.2016.09.023",
"doi-asserted-by": "publisher",
"key": "ref11"
},
{
"DOI": "10.3389/fimmu.2020.570122",
"doi-asserted-by": "publisher",
"key": "ref12"
},
{
"DOI": "10.1016/j.nut.2020.110989",
"doi-asserted-by": "publisher",
"key": "ref13"
},
{
"DOI": "10.3389/fimmu.2020.01712",
"doi-asserted-by": "publisher",
"key": "ref14"
},
{
"DOI": "10.3390/nu10091203",
"doi-asserted-by": "publisher",
"key": "ref15"
},
{
"DOI": "10.3390/nu9121286",
"doi-asserted-by": "publisher",
"key": "ref16"
},
{
"DOI": "10.1016/j.abb.2016.03.022",
"doi-asserted-by": "publisher",
"key": "ref17"
},
{
"DOI": "10.3945/an.114.007575",
"doi-asserted-by": "publisher",
"key": "ref18"
},
{
"DOI": "10.3390/nu11092101",
"doi-asserted-by": "publisher",
"key": "ref19"
},
{
"DOI": "10.3390/nu12072098",
"doi-asserted-by": "publisher",
"key": "ref20"
},
{
"DOI": "10.1016/j.redox.2020.101764",
"doi-asserted-by": "publisher",
"key": "ref21"
},
{
"DOI": "10.7326/0003-4819-147-8-200710160-00010-w1",
"doi-asserted-by": "publisher",
"key": "ref22"
},
{
"DOI": "10.2807/1560-7917.ES.2020.25.3.2000045",
"doi-asserted-by": "publisher",
"key": "ref23"
},
{
"DOI": "10.1016/s0076-6879(84)05015-1",
"doi-asserted-by": "publisher",
"key": "ref24"
},
{
"DOI": "10.1002/ijc.29071",
"doi-asserted-by": "publisher",
"key": "ref25"
},
{
"DOI": "10.1093/carcin/bgx051",
"doi-asserted-by": "publisher",
"key": "ref26"
},
{
"DOI": "10.1001/jama.2012.5669",
"doi-asserted-by": "publisher",
"key": "ref27"
},
{
"DOI": "10.1002/cyto.b.20432",
"doi-asserted-by": "publisher",
"key": "ref28"
},
{
"DOI": "10.1111/j.1365-2249.2010.04206.x",
"doi-asserted-by": "publisher",
"key": "ref29"
},
{
"DOI": "10.1001/jamainternmed.2020.3539",
"doi-asserted-by": "publisher",
"key": "ref30"
},
{
"DOI": "10.1056/NEJMoa2004500",
"doi-asserted-by": "publisher",
"key": "ref31"
},
{
"DOI": "10.1001/jama.2020.4326",
"doi-asserted-by": "publisher",
"key": "ref32"
},
{
"DOI": "10.1016/S2213-2600(20)30079-5",
"doi-asserted-by": "publisher",
"key": "ref33"
},
{
"DOI": "10.1016/j.immuni.2020.05.002",
"doi-asserted-by": "publisher",
"key": "ref34"
},
{
"DOI": "10.1097/01.CCM.0000055371.27268.36",
"doi-asserted-by": "publisher",
"key": "ref35"
},
{
"DOI": "10.1097/00003246-199809000-00021",
"doi-asserted-by": "publisher",
"key": "ref36"
},
{
"DOI": "10.1007/s00134-008-1356-5",
"doi-asserted-by": "publisher",
"key": "ref37"
},
{
"DOI": "10.1080/08820139.2018.1496098",
"doi-asserted-by": "publisher",
"key": "ref38"
},
{
"DOI": "10.1097/01.CCM.0000251124.83436.0E",
"doi-asserted-by": "publisher",
"key": "ref39"
},
{
"DOI": "10.1056/NEJMoa1212722",
"doi-asserted-by": "publisher",
"key": "ref40"
},
{
"DOI": "10.1002/14651858.CD003703.pub3",
"doi-asserted-by": "publisher",
"key": "ref41"
},
{
"DOI": "10.1186/s13054-016-1529-5",
"doi-asserted-by": "publisher",
"key": "ref42"
},
{
"DOI": "10.1371/journal.pone.0054431",
"doi-asserted-by": "publisher",
"key": "ref43"
},
{
"DOI": "10.1016/j.clnu.2018.08.037",
"doi-asserted-by": "publisher",
"key": "ref44"
},
{
"DOI": "10.1074/jbc.M202773200",
"doi-asserted-by": "publisher",
"key": "ref45"
},
{
"DOI": "10.1016/j.jnutbio.2012.06.007",
"doi-asserted-by": "publisher",
"key": "ref46"
},
{
"DOI": "10.1093/ajcn/68.2.447S",
"doi-asserted-by": "publisher",
"key": "ref47"
},
{
"DOI": "10.1093/cid/ciaa641",
"doi-asserted-by": "publisher",
"key": "ref48"
},
{
"DOI": "10.1016/j.jcv.2020.104370",
"doi-asserted-by": "publisher",
"key": "ref49"
},
{
"DOI": "10.1016/S2213-2600(20)30404-5",
"doi-asserted-by": "publisher",
"key": "ref50"
},
{
"DOI": "10.1039/D0MT00165A",
"doi-asserted-by": "publisher",
"key": "ref51"
},
{
"DOI": "10.1039/C5MT00211G",
"doi-asserted-by": "publisher",
"key": "ref52"
},
{
"DOI": "10.1016/j.cytogfr.2011.10.001",
"doi-asserted-by": "publisher",
"key": "ref53"
},
{
"DOI": "10.1038/s41423-020-0402-2",
"doi-asserted-by": "publisher",
"key": "ref54"
},
{
"DOI": "10.1182/blood-2005-02-0456",
"doi-asserted-by": "publisher",
"key": "ref55"
},
{
"DOI": "10.3389/fimmu.2021.661052",
"doi-asserted-by": "publisher",
"key": "ref56"
},
{
"DOI": "10.1371/journal.pone.0242917",
"doi-asserted-by": "publisher",
"key": "ref57"
},
{
"DOI": "10.3389/fimmu.2020.581338",
"doi-asserted-by": "publisher",
"key": "ref58"
},
{
"DOI": "10.3390/nu12010236",
"doi-asserted-by": "publisher",
"key": "ref59"
},
{
"DOI": "10.1378/chest.08-2408",
"doi-asserted-by": "publisher",
"key": "ref60"
},
{
"DOI": "10.1016/j.nut.2012.05.013",
"doi-asserted-by": "publisher",
"key": "ref61"
},
{
"DOI": "10.1016/j.clnu.2017.04.019",
"doi-asserted-by": "publisher",
"key": "ref62"
},
{
"DOI": "10.1186/cc5960",
"doi-asserted-by": "publisher",
"key": "ref63"
},
{
"DOI": "10.1155/2017/7478523",
"doi-asserted-by": "publisher",
"key": "ref64"
}
],
"reference-count": 64,
"references-count": 64,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2072-6643/13/6/2113"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Clinical Significance of Micronutrient Supplementation in Critically Ill COVID-19 Patients with Severe ARDS",
"type": "journal-article",
"volume": "13"
}
