A phase 2 randomized, double-blinded, placebo-controlled, multicenter trial evaluating the efficacy and safety of raloxifene for patients with mild to moderate COVID-19
et al., eClinicalMedicine, doi:10.1016/j.eclinm.2022.101450, NCT05172050, Jun 2022
7th treatment shown to reduce risk in
September 2020, now with p = 0.000000056 from 49 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 68 patients in Italy showing improved viral clearance with raloxifene.
|
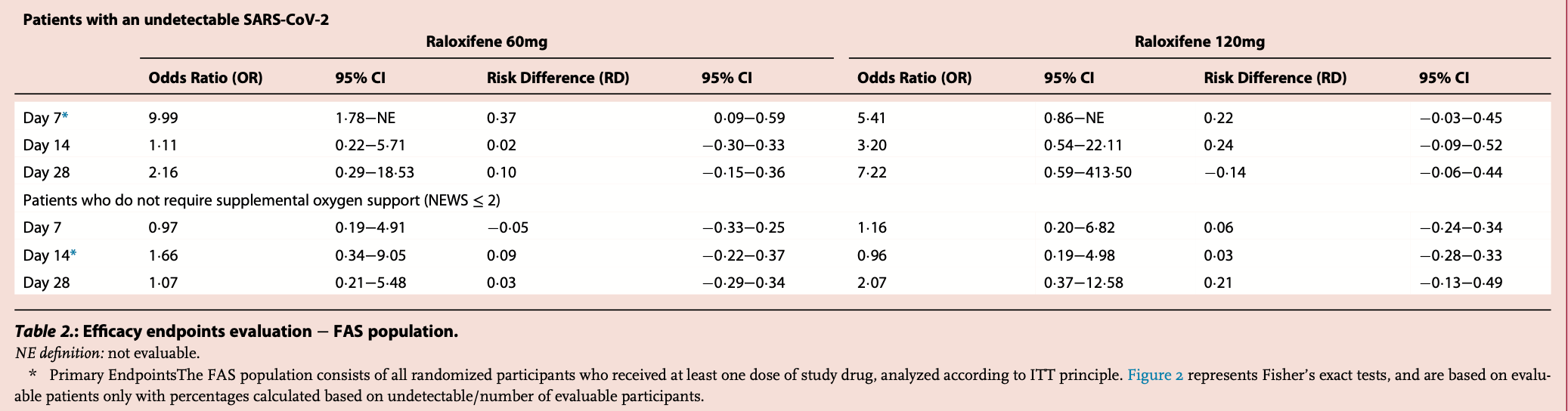
risk of oxygen therapy, 51.7% lower, OR 0.48, p = 0.43, treatment 20, control 19, inverted to make OR<1 favor treatment, oxygen supplementation or mechanical ventilation, day 28, 120mg, RR approximated with OR.
|
|
risk of oxygen therapy, 6.5% lower, OR 0.93, p = 0.94, treatment 22, control 19, inverted to make OR<1 favor treatment, oxygen supplementation or mechanical ventilation, day 28, 60mg, RR approximated with OR.
|
|
risk of oxygen therapy, 4.2% higher, OR 1.04, p = 0.96, treatment 20, control 19, inverted to make OR<1 favor treatment, oxygen supplementation or mechanical ventilation, day 14, 120mg, primary outcome, RR approximated with OR.
|
|
risk of oxygen therapy, 39.8% lower, OR 0.60, p = 0.56, treatment 22, control 19, inverted to make OR<1 favor treatment, oxygen supplementation or mechanical ventilation, day 14, 60mg, primary outcome, RR approximated with OR.
|
|
risk of no viral clearance, 68.8% lower, OR 0.31, p = 0.22, treatment 20, control 19, inverted to make OR<1 favor treatment, mid-recovery, day 14, 120mg, RR approximated with OR.
|
|
risk of no viral clearance, 9.9% lower, OR 0.90, p = 0.91, treatment 22, control 19, inverted to make OR<1 favor treatment, mid-recovery, day 14, 60mg, RR approximated with OR.
|
|
risk of no viral clearance, 81.5% lower, OR 0.18, treatment 20, control 19, inverted to make OR<1 favor treatment, mid-recovery, day 7, 120mg, primary outcome, RR approximated with OR.
|
|
risk of no viral clearance, 90.0% lower, OR 0.10, treatment 22, control 19, inverted to make OR<1 favor treatment, mid-recovery, day 7, 60mg, primary outcome, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Nicastri et al., 30 Jun 2022, Double Blind Randomized Controlled Trial, placebo-controlled, Italy, peer-reviewed, 17 authors, study period October 2020 - June 2021, trial NCT05172050 (history).
Contact: flavio.mantelli@dompe.com.
A phase 2 randomized, double-blinded, placebo-controlled, multicenter trial evaluating the efficacy and safety of raloxifene for patients with mild to moderate COVID-19
eClinicalMedicine, doi:10.1016/j.eclinm.2022.101450
Background Current available therapeutic options for Coronavirus Disease-2019 (COVID-19) are primarily focused on treating hospitalized patients, and there is a lack of oral therapeutic options to treat mild to moderate outpatient COVID-19 and prevent clinical progression. Raloxifene was found as a promising molecule to treat COVID-19 due to its activity to modulate the replication of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) and act as an immunomodulator to decrease proinflammatory cytokines. Methods This was a phase 2 multicenter, randomized, placebo-controlled trial to evaluate the efficacy and safety of raloxifene in adult patients with mild to moderate COVID-19 between October 2020 to June 2021 in five centers located in Italy. This was a planned 2/3 adaptive study, but due to operational difficulties, the study was discontinued during the phase 2 study segment. Participants were randomized 1:1:1 to receive oral placebo, raloxifene 60 mg, or raloxifene 120 mg by self-administration for a maximum of two weeks. The primary outcomes were the proportion of patients with undetectable SARS-CoV-2 via nasopharyngeal swabs at day 7 and the proportion of patients who did not require supplemental oxygen therapy or mechanical ventilation on day 14. Safety was assessed. The trial is registered (2020-003936-25 and ClinicalTrials.gov: NCT05172050). Findings A total of 68 participants were enrolled and randomized to placebo (n = 21), raloxifene 60 mg (n = 24), and raloxifene 120 mg (n = 23). The proportion of participants with undetectable SARS-CoV-2 after seven days of treatment with raloxifene 60 mg [36.8%, 7/19 vs. 0.0%, 0/14] and 120 mg [22.2%, 4/18 vs. 0.0%, 0/14] was better compared to placebo, [risk difference (RD) = 0¢37 (95% C.I.:0¢09−0¢59)] and [RD = 0¢22 (95% C.I.: -0¢03−0¢45)], respectively. There was no evidence of effect for requirement of supplemental oxygen and/or mechanical ventilation with effects for raloxifene 60 mg and raloxifene 120 mg over placebo, [RD = 0¢09 (95% C.I.: -0¢22−0¢37)], and [RD = 0¢03 (95% C.I.: -0¢28−0¢33)], respectively. Raloxifene was well tolerated at both doses, and there was no evidence of any difference in the occurrence of serious adverse events. Interpretation Raloxifene showed evidence of effect in the primary virologic endpoint in the treatment of early mild to moderate COVID-19 patients shortening the time of viral shedding. The safety profile was consistent with that reported for other indications. Raloxifene may represent a promising pharmacological option to prevent or mitigate COVID-19 disease progression.
Muscle or body aches 10 (52¢6% Table 1 : Patient baseline characteristics − FAS/SAF population. Data are presented for the FAS as number, proportion (%) with 95% exact C.I. or mean § SD (standard deviation) Sp02:Oxygen Saturation. * All centers were located in Italy. a Anticoagulants included heparin and enoxaparin. 1 Antibiotics include pencillins, fluroquinolones, and macrolides. x Corticosteroids included dexamethasone and prednisone,This clinical trial was impacted due to the COVID-19 pandemic, and experienced operational difficulties for patient enrollment. The following modifications were made to the original patient population to increase patient recruitment: inclusion of additional centers, lowering the age of the inclusion criteria from 50 to 40 years of age, increasing the time window of SARS-CoV-2 positive results from 7 to 10 days, and deleting a time cut-off of when initial COVID-19 related symptoms were experienced by patients (fever, dyspnea, headache, cough, dysgeusia, conjunctivitis, vomiting, diarrhea, anosmia, muscle or body aches or other symptoms). Any adverse event (Grade ≥3) Adverse Events of Special Interest (AESI) All data are presented as number of patients, proportion (%) with 95% exact C.I. Abbreviations: TEAE: treatment-emergent adverse events, TESAE: Treatment-emergent serious adverse events. Venous
Declaration of interests
Supplementary materials Supplementary material associated with this article can be found in the online version at..
References
Adomaityte, Farooq, Qayyum, Effect of raloxifene therapy on venous thromboembolism in postmenopausal women. A metaanalysis, Thromb Haemost
Allegretti, Cesta, Zippoli, Repurposing the estrogen receptor modulator raloxifene to treat SARS-CoV-2 infection, Cell Death Differ
Ciceri, Ruggeri, Lembo, Puglisi, Landoni et al., Decreased in-hospital mortality in patients with COVID-19 pneumonia, Pathog Glob Health
Doran, Riggs, Atkinson, Khosla, Effects of raloxifene, a selective estrogen receptor modulator, on bone turnover markers and serum sex steroid and lipid levels in elderly men, J Bone Miner Res
Duschek, Gooren, Netelenbos, Effects of raloxifene on gonadotrophins, sex hormones, bone turnover and lipids in healthy elderly men, Eur J Endocrinol
Enga, Braekkan, Hansen-Krone, Le Cessie, Rosendaal et al., Cigarette smoking and the risk of venous thromboembolism: the Tromsø study, J Thromb Haemost JTH
Eyre, Kirby, Anfiteatro, Identification of estrogen receptor modulators as inhibitors of flavivirus infection, Antimicrob Agents Chemother
Fajgenbaum, June, Cytokine storm, N Engl J Med
Fuentes, Silveyra, Estrogen receptor signaling mechanisms, Adv Protein Chem Struct Biol
Gianni, Ricci, Gazzaniga, Raloxifene modulates interleukin-6 and tumor necrosis factor-alpha synthesis in vivo: results from a pilot clinical study, J Clin Endocrinol Metab
Hern Andez, Valera, Alonzo, Effects of raloxifene on bone metabolism and serum lipids in postmenopausal women on chronic hemodialysis, Kidney Int
Hess, Cooke, Estrogen in the male: a historical perspective, Biol Reprod
Hong, Chang, Jeong, Lee, Raloxifene as a treatment option for viral infections, J Microbiol
Iaconis, Talarico, Manelfi, Characterization of raloxifene as potential pharmacological agent against SARS-CoV-2 and its variants, bioRxiv
Jin, Yang, Chen, Zhang, Duan, Endothelial activation and dysfunction in COVID-19: from basic mechanisms to potential therapeutic approaches, Signal Transduct Target Ther
Lewis, Jordan, Selective estrogen receptor modulators (SERMs): mechanisms of anticarcinogenesis and drug resistance, Mutat Res
Li, Jerkic, Slutsky, Zhang, Molecular mechanisms of sex bias differences in COVID-19 mortality, Crit Care
Libby, COVID-19 is, in the end, an endothelial disease, Eur Heart J
Moores, Tritschler, Brosnahan, Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019: CHEST guideline and expert panel report, Chest
Patel, Bihani, Selective estrogen receptor modulators (SERMs) and selective estrogen receptor degraders (SERDs) in cancer treatment, Pharmacol Ther
Peckham, De Gruijter, Raine, Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission, Nat Commun
Redaelli, Landoni, Napoli, Novel coronavirus disease (COVID-19) in Italian patients: gender differences in presentation and severity, Saudi J Med Med Sci
Solinas, Perra, Aiello, Migliori, Petrosillo, A critical evaluation of glucocorticoids in the management of severe COVID-19, Cytokine Growth Factor Rev
Stelzig, Canepa-Escaro, Schiliro, Berdnikovs, Prakash et al., Estrogen regulates the expression of SARS-CoV-2 receptor ACE2 in differentiated airway epithelial cells, Am J Physiol Lung Cell Mol Physiol
Weinreich, Sivapalasingam, Norton, REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19, N Engl J Med
Wu, Mcgoogan, Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the chinese center for disease control and prevention, JAMA
DOI record:
{
"DOI": "10.1016/j.eclinm.2022.101450",
"ISSN": [
"2589-5370"
],
"URL": "http://dx.doi.org/10.1016/j.eclinm.2022.101450",
"alternative-id": [
"S2589537022001808"
],
"article-number": "101450",
"author": [
{
"affiliation": [],
"family": "Nicastri",
"given": "Emanuele",
"sequence": "first"
},
{
"affiliation": [],
"family": "Marinangeli",
"given": "Franco",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pivetta",
"given": "Emanuele",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Torri",
"given": "Elena",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Reggiani",
"given": "Francesco",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fiorentino",
"given": "Giuseppe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Scorzolini",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vettori",
"given": "Serena",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4510-9582",
"affiliation": [],
"authenticated-orcid": false,
"family": "Marsiglia",
"given": "Carolina",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6436-0748",
"affiliation": [],
"authenticated-orcid": false,
"family": "Gavioli",
"given": "Elizabeth Marie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Beccari",
"given": "Andrea R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Terpolilli",
"given": "Giuseppe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "De Pizzol",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Goisis",
"given": "Giovanni",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mantelli",
"given": "Flavio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vaia",
"given": "Francesco",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Allegretti",
"given": "Marcello",
"sequence": "additional"
}
],
"container-title": "eClinicalMedicine",
"container-title-short": "eClinicalMedicine",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
5,
12
]
],
"date-time": "2022-05-12T22:42:03Z",
"timestamp": 1652395323000
},
"deposited": {
"date-parts": [
[
2022,
5,
12
]
],
"date-time": "2022-05-12T22:42:23Z",
"timestamp": 1652395343000
},
"indexed": {
"date-parts": [
[
2022,
5,
12
]
],
"date-time": "2022-05-12T23:11:49Z",
"timestamp": 1652397109318
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
6
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
6,
1
]
],
"date-time": "2022-06-01T00:00:00Z",
"timestamp": 1654041600000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
4,
26
]
],
"date-time": "2022-04-26T00:00:00Z",
"timestamp": 1650931200000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2589537022001808?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2589537022001808?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "101450",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
6
]
]
},
"published-print": {
"date-parts": [
[
2022,
6
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"key": "10.1016/j.eclinm.2022.101450_bib0001",
"unstructured": "World Health Organization. Listings of WHO's response to COVID-19. Accessed August 17, 2021. Available at: https://www.who.int/news/item/29-06-2020-covidtimeline."
},
{
"DOI": "10.1001/jama.2020.2648",
"article-title": "Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the chinese center for disease control and prevention",
"author": "Wu",
"doi-asserted-by": "crossref",
"first-page": "1239",
"issue": "13",
"journal-title": "JAMA",
"key": "10.1016/j.eclinm.2022.101450_bib0002",
"volume": "323",
"year": "2020"
},
{
"key": "10.1016/j.eclinm.2022.101450_bib0003",
"series-title": "People with Certain Medical Conditions",
"year": "2021"
},
{
"DOI": "10.1093/eurheartj/ehaa623",
"article-title": "COVID-19 is, in the end, an endothelial disease",
"author": "Libby",
"doi-asserted-by": "crossref",
"first-page": "3038",
"issue": "32",
"journal-title": "Eur Heart J",
"key": "10.1016/j.eclinm.2022.101450_bib0004",
"volume": "41",
"year": "2020"
},
{
"DOI": "10.1056/NEJMra2026131",
"article-title": "Cytokine storm",
"author": "Fajgenbaum",
"doi-asserted-by": "crossref",
"first-page": "2255",
"issue": "23",
"journal-title": "N Engl J Med",
"key": "10.1016/j.eclinm.2022.101450_bib0005",
"volume": "383",
"year": "2020"
},
{
"key": "10.1016/j.eclinm.2022.101450_bib0006",
"unstructured": "COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available at https://www.covid19treatmentguidelines.nih.gov/ Accessed 27 August 2021."
},
{
"DOI": "10.1038/s41467-020-19741-6",
"article-title": "Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission",
"author": "Peckham",
"doi-asserted-by": "crossref",
"first-page": "6317",
"issue": "1",
"journal-title": "Nat Commun",
"key": "10.1016/j.eclinm.2022.101450_bib0007",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1186/s13054-020-03118-8",
"article-title": "Molecular mechanisms of sex bias differences in COVID-19 mortality",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "405",
"issue": "1",
"journal-title": "Crit Care",
"key": "10.1016/j.eclinm.2022.101450_bib0008",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1152/ajplung.00153.2020",
"article-title": "Estrogen regulates the expression of SARS-CoV-2 receptor ACE2 in differentiated airway epithelial cells",
"author": "Stelzig",
"doi-asserted-by": "crossref",
"first-page": "L1280",
"issue": "6",
"journal-title": "Am J Physiol Lung Cell Mol Physiol",
"key": "10.1016/j.eclinm.2022.101450_bib0009",
"volume": "318",
"year": "2020"
},
{
"article-title": "Repurposing the estrogen receptor modulator raloxifene to treat SARS-CoV-2 infection",
"author": "Allegretti",
"journal-title": "Cell Death Differ",
"key": "10.1016/j.eclinm.2022.101450_bib0010",
"year": "2021"
},
{
"key": "10.1016/j.eclinm.2022.101450_bib0011",
"unstructured": "EVISTA (raloxifene hydrochloride). Package insert. Lilly USA, LLC, Indianapolis, IN; 1997. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/020815s034lbl.pdf. Accessed 5 May 2020"
},
{
"DOI": "10.1016/j.pharmthera.2017.12.012",
"article-title": "Selective estrogen receptor modulators (SERMs) and selective estrogen receptor degraders (SERDs) in cancer treatment",
"author": "Patel",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Pharmacol Ther",
"key": "10.1016/j.eclinm.2022.101450_bib0012",
"volume": "186",
"year": "2018"
},
{
"DOI": "10.1007/s12275-021-0617-7",
"article-title": "Raloxifene as a treatment option for viral infections",
"author": "Hong",
"doi-asserted-by": "crossref",
"first-page": "124",
"issue": "2",
"journal-title": "J Microbiol",
"key": "10.1016/j.eclinm.2022.101450_bib0013",
"volume": "59",
"year": "2021"
},
{
"DOI": "10.1128/AAC.00289-20",
"article-title": "Identification of estrogen receptor modulators as inhibitors of flavivirus infection",
"author": "Eyre",
"doi-asserted-by": "crossref",
"issue": "8",
"journal-title": "Antimicrob Agents Chemother",
"key": "10.1016/j.eclinm.2022.101450_bib0014",
"volume": "64",
"year": "2020"
},
{
"DOI": "10.1210/jc.2004-0795",
"article-title": "Raloxifene modulates interleukin-6 and tumor necrosis factor-alpha synthesis in vivo: results from a pilot clinical study",
"author": "Gianni",
"doi-asserted-by": "crossref",
"first-page": "6097",
"issue": "12",
"journal-title": "J Clin Endocrinol Metab",
"key": "10.1016/j.eclinm.2022.101450_bib0015",
"volume": "89",
"year": "2004"
},
{
"DOI": "10.1038/s41392-020-00454-7",
"article-title": "Endothelial activation and dysfunction in COVID-19: from basic mechanisms to potential therapeutic approaches",
"author": "Jin",
"doi-asserted-by": "crossref",
"first-page": "293",
"issue": "1",
"journal-title": "Signal Transduct Target Ther",
"key": "10.1016/j.eclinm.2022.101450_bib0016",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1016/j.mrfmmm.2005.02.028",
"article-title": "Selective estrogen receptor modulators (SERMs): mechanisms of anticarcinogenesis and drug resistance",
"author": "Lewis",
"doi-asserted-by": "crossref",
"first-page": "247",
"issue": "1–2",
"journal-title": "Mutat Res",
"key": "10.1016/j.eclinm.2022.101450_bib0017",
"volume": "591",
"year": "2005"
},
{
"DOI": "10.1016/bs.apcsb.2019.01.001",
"article-title": "Estrogen receptor signaling mechanisms",
"author": "Fuentes",
"doi-asserted-by": "crossref",
"first-page": "135",
"journal-title": "Adv Protein Chem Struct Biol",
"key": "10.1016/j.eclinm.2022.101450_bib0018",
"volume": "116",
"year": "2019"
},
{
"DOI": "10.1093/biolre/ioy043",
"article-title": "Estrogen in the male: a historical perspective",
"author": "Hess",
"doi-asserted-by": "crossref",
"first-page": "27",
"issue": "1",
"journal-title": "Biol Reprod",
"key": "10.1016/j.eclinm.2022.101450_bib0019",
"volume": "99",
"year": "2018"
},
{
"DOI": "10.4103/sjmms.sjmms_542_20",
"article-title": "Novel coronavirus disease (COVID-19) in Italian patients: gender differences in presentation and severity",
"author": "Baiardo Redaelli",
"doi-asserted-by": "crossref",
"first-page": "59",
"issue": "1",
"journal-title": "Saudi J Med Med Sci",
"key": "10.1016/j.eclinm.2022.101450_bib0020",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1016/j.cytogfr.2020.06.012",
"article-title": "A critical evaluation of glucocorticoids in the management of severe COVID-19",
"author": "Solinas",
"doi-asserted-by": "crossref",
"first-page": "8",
"journal-title": "Cytokine Growth Factor Rev",
"key": "10.1016/j.eclinm.2022.101450_bib0021",
"volume": "54",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2035002",
"article-title": "REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19",
"author": "Weinreich",
"doi-asserted-by": "crossref",
"first-page": "238",
"issue": "3",
"journal-title": "N Engl J Med",
"key": "10.1016/j.eclinm.2022.101450_bib0022",
"volume": "384",
"year": "2020"
},
{
"key": "10.1016/j.eclinm.2022.101450_bib0023",
"unstructured": "U. S. Food and drug administration. Corona virus (COVID-19) update: FDA revokes emergency use authorization for monoclonal antibody bamlanivimab. April 16, 2021."
},
{
"article-title": "Characterization of raloxifene as potential pharmacological agent against SARS-CoV-2 and its variants",
"author": "Iaconis",
"journal-title": "bioRxiv",
"key": "10.1016/j.eclinm.2022.101450_bib0024",
"year": "2021"
},
{
"DOI": "10.1359/jbmr.2001.16.11.2118",
"article-title": "Effects of raloxifene, a selective estrogen receptor modulator, on bone turnover markers and serum sex steroid and lipid levels in elderly men",
"author": "Doran",
"doi-asserted-by": "crossref",
"first-page": "2118",
"issue": "11",
"journal-title": "J Bone Miner Res",
"key": "10.1016/j.eclinm.2022.101450_bib0025",
"volume": "16",
"year": "2001"
},
{
"DOI": "10.1530/eje.0.1500539",
"article-title": "Effects of raloxifene on gonadotrophins, sex hormones, bone turnover and lipids in healthy elderly men",
"author": "Duschek",
"doi-asserted-by": "crossref",
"first-page": "539",
"issue": "4",
"journal-title": "Eur J Endocrinol",
"key": "10.1016/j.eclinm.2022.101450_bib0026",
"volume": "150",
"year": "2004"
},
{
"DOI": "10.1160/TH07-07-0468",
"article-title": "Effect of raloxifene therapy on venous thromboembolism in postmenopausal women. A meta-analysis",
"author": "Adomaityte",
"doi-asserted-by": "crossref",
"first-page": "338",
"issue": "2",
"journal-title": "Thromb Haemost",
"key": "10.1016/j.eclinm.2022.101450_bib0027",
"volume": "99",
"year": "2008"
},
{
"DOI": "10.1016/j.chest.2020.05.559",
"article-title": "Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019: CHEST guideline and expert panel report",
"author": "Moores",
"doi-asserted-by": "crossref",
"first-page": "1143",
"issue": "3",
"journal-title": "Chest",
"key": "10.1016/j.eclinm.2022.101450_bib0028",
"volume": "158",
"year": "2020"
},
{
"DOI": "10.1111/j.1538-7836.2012.04880.x",
"article-title": "Cigarette smoking and the risk of venous thromboembolism: the Tromsø study",
"author": "Enga",
"doi-asserted-by": "crossref",
"first-page": "2068",
"issue": "10",
"journal-title": "J Thromb Haemost JTH",
"key": "10.1016/j.eclinm.2022.101450_bib0029",
"volume": "10",
"year": "2012"
},
{
"DOI": "10.1046/j.1523-1755.2003.00005.x",
"article-title": "Effects of raloxifene on bone metabolism and serum lipids in postmenopausal women on chronic hemodialysis",
"author": "Hernández",
"doi-asserted-by": "crossref",
"first-page": "2269",
"issue": "6",
"journal-title": "Kidney Int",
"key": "10.1016/j.eclinm.2022.101450_bib0030",
"volume": "63",
"year": "2003"
},
{
"DOI": "10.1080/20477724.2020.1785782",
"article-title": "Decreased in-hospital mortality in patients with COVID-19 pneumonia",
"author": "Ciceri",
"doi-asserted-by": "crossref",
"first-page": "281",
"issue": "6",
"journal-title": "Pathog Glob Health",
"key": "10.1016/j.eclinm.2022.101450_bib0031",
"volume": "114",
"year": "2020"
}
],
"reference-count": 31,
"references-count": 31,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2589537022001808"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "A phase 2 randomized, double-blinded, placebo-controlled, multicenter trial evaluating the efficacy and safety of raloxifene for patients with mild to moderate COVID-19",
"type": "journal-article",
"volume": "48"
}
