The short-term effect of different chlorhexidine forms versus povidone iodine mouth rinse in minimizing the oral SARS-CoV-2 viral load: An open label randomized controlled clinical trial study
et al., Medicine, doi:10.1097/MD.0000000000028925, NCT04941131, Jul 2022
PVP-I for COVID-19
15th treatment shown to reduce risk in
February 2021, now with p = 0.000000000016 from 22 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
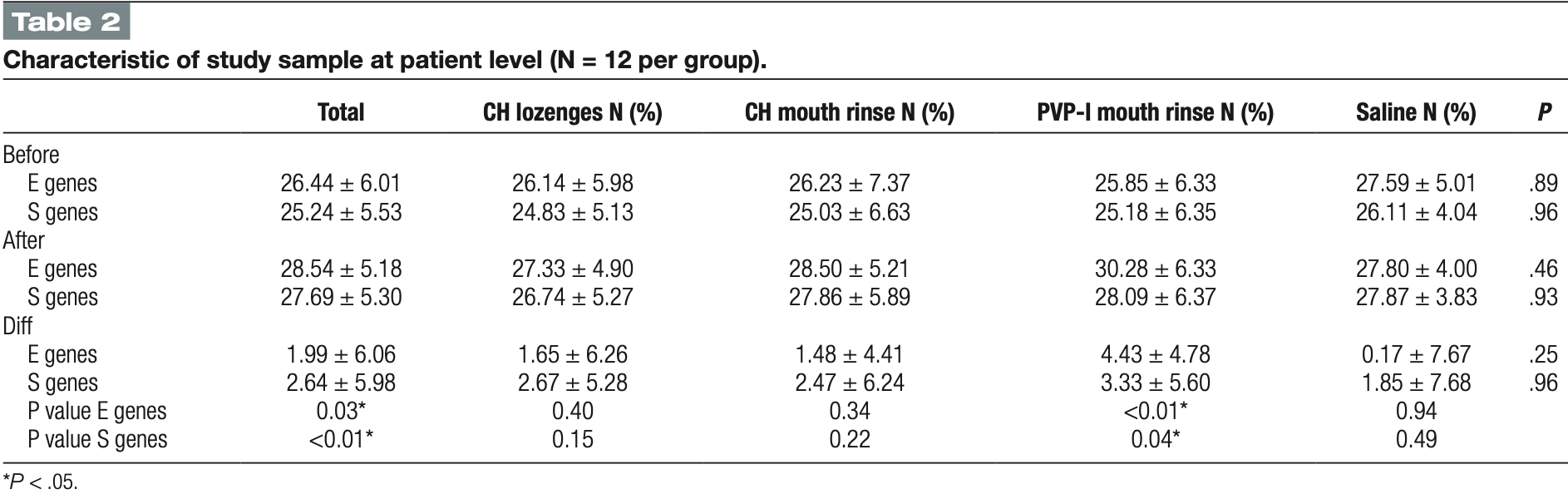
60 patient RCT comparing chlorhexidine, PVP-I, and saline in Saudi Arabia with a single mouth rinse treatment and PCR testing 5 minutes later, showing statistically significant improvement in Ct value for PVP-I. PVP-I showed greater improvement than saline, without statistical significance.
Analysis of short-term changes in viral load using PCR may not detect
effective treatments because PCR is unable to differentiate between intact
infectious virus and non-infectious or destroyed virus particles. For example
Tarragó-Gil, Alemany perform RCTs with cetylpyridinium chloride
(CPC) mouthwash that show no difference in PCR viral load, however there was
significantly increased detection of SARS-CoV-2 nucleocapsid protein,
indicating viral lysis. CPC inactivates SARS-CoV-2 by degrading its membrane,
exposing the nucleocapsid of the virus. To better estimate changes in viral
load and infectivity, methods like viral culture that can
differentiate intact vs. degraded virus are preferred.
This study is excluded in the after exclusion results of meta-analysis:
study only provides short-term viral load results.
Study covers chlorhexidine and povidone-iodine.
|
risk of viral load, 73.6% lower, RR 0.26, p = 0.27, treatment 12, control 12, relative improvement in Ct value, both genes combined.
|
|
risk of viral load, 96.2% lower, RR 0.04, p = 0.12, treatment mean 4.43 (±4.78) n=12, control mean 0.17 (±7.67) n=12, relative improvement in Ct value, E gene.
|
|
risk of viral load, 44.4% lower, RR 0.56, p = 0.60, treatment mean 3.33 (±5.6) n=12, control mean 1.85 (±7.68) n=12, relative improvement in Ct value, S gene.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Natto et al., 29 Jul 2022, Randomized Controlled Trial, Saudi Arabia, peer-reviewed, 7 authors, study period June 2021 - July 2021, this trial compares with another treatment - results may be better when compared to placebo, trial NCT04941131 (history).
The short-term effect of different chlorhexidine forms versus povidone iodine mouth rinse in minimizing the oral SARS-CoV-2 viral load: An open label randomized controlled clinical trial study
Medicine, doi:10.1097/md.0000000000028925
Several investigations evaluated the possibility of different types of mouth wash rinse in minimizing the SARS-CoV-2 load. However, results still controversial. The study aim is to assess the short-term efficiency of several over-the-counter mouth rinses and lozenges in minimizing the salivary viral load for SARS-CoV-2 in patients with confirmed COVID-19 in comparison to saline. This is a randomized controlled clinical trial with 4 arms. The recruited cases were randomized using a simple randomization technique and were assigned to chlorhexidine digluconate mouth rinse (CHX mouth rinse), 2 mg of chlorhexidine digluconate lozenges (CHX lozenges), povidone iodine mouth rinse (PVP-I mouth rinse) or saline as a control group. Saliva were collected from all study subjects by passive drool technique at two time points. First, prior to intervention with mouth rinse or the lozenges, the baseline saliva sample was collected. Second saliva samples were collected immediately after the mouth rinse. Real time PCR was conducted and the value threshold cycle (Ct) for each sample was recorded. Majority of the participants had an education level of high school or less (60%), were married (68.3), males (58.3%), and nonsmokers (58.5%). No statistically significant differences between groups at the two times test (P > .05). However, a significant decrease of salivary viral load in all four groups combined (P-value for E genes = .027, and for S genes = .006), and in PVP-I mouth rinse specifically (P = .003 and P = .045, respectively). Povidone iodine mouth rinse showed a potential influence on the reduction of the viral load on a short-term basis. However, longer-term studies of the effect of these products should be conducted. Abbreviations: CHX mouth rinse = digluconate mouth rinse, CHX lozenges = chlorhexidine digluconate lozenges, PVP-I mouth rinse = povidone iodine mouth rinse, Ct = threshold cycle, SARS-CoV-2 = Severe Acute Respiratory Syndrome Coronavirus 2, KFGH = King Fahad General Hospital, RT-PCR = Real-time polymerase chain reaction.
References
Alene, Yismaw, Assemie, Ketema, Gietaneh et al., Serial interval and incubation period of COVID-19: a systematic review and meta-analysis, BMC Infect Dis
Alharthi, Natto, Midle, Gyurko, Neill et al., Association between time since quitting smoking and periodontitis in former smokers in the National Health and Nutrition Examination Surveys (NHANES) 2009 to 2012, J Periodontol
Alshaeri, Natto, A contemporary look at COVID-19 medications: available and potentially effective drugs, Eur Rev Med Pharmacol Sci
Ather, Patel, Ruparel, Diogenes, Hargreaves, Coronavirus disease 19 (COVID-19): implications for clinical dental care, J Endod
Baqui, Kelley, Jabra-Rizk, Depaola, Falkler et al., In vitro effect of oral antiseptics on human immunodeficiency virus-1 and herpes simplex virus type 1, J Clin Periodontol
Bernstein, Schiff, Echler, Prince, Feller et al., In vitro virucidal effectiveness of a 0.12%-chlorhexidine gluconate mouthrinse, J Dent Res
Biadsee, Kassem, Dagan, Masarwa, Ormianer, Olfactory and oral manifestations of COVID-19: sex-related symptoms-a potential pathway to early diagnosis, Otolaryngol Head Neck Surg
Bidra, Pelletier, Westover, Frank, Brown et al., Rapid in-vitro inactivation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) using povidone-iodine oral antiseptic rinse, J Prosthodont
Cascella, Rajnik, Aleem, Dulebohn, Napoli, Features, evaluation and treatment coronavirus (COVID-19)
Challacombe, Kirk-Bayley, Sunkaraneni, Combes, Povidone iodine, Br Dent J
Chopra, Sivaraman, Radhakrishnan, Balakrishnan, Narayana, Can povidone iodine gargle/mouthrinse inactivate SARS-CoV-2 and decrease the risk of nosocomial and community transmission during the COVID-19 pandemic? An evidence-based update, Jpn Dent Sci Rev
Eduardo, Corrêa, Heller, Salivary SARS-CoV-2 load reduction with mouthwash use: a randomized pilot clinical trial, Heliyon
Eggers, Infectious disease management and control with povidone iodine, Infect Dis Ther
Elzein, Sater, Fakhreddine, In vivo evaluation of the virucidal efficacy of chlorhexidine and povidone-iodine mouthwashes against salivary SARS-CoV-2. A randomized-controlled clinical trial, J Evid Based Dent Pract
Helmi, Alosaimy, Goodson, Hasturk, Natto, Annual alveolar bone loss in older adults taking oral bisphosphonate: a retrospective cohort study, BMC Oral Health
Helmi, Goodson, Hasturk, Natto, Annual alveolar bone loss in subjects with cardiovascular disease adjusting for associated systemic diseases and risk factors: a retrospective study, BMC Oral Health
Helmi, Huang, Goodson, Hasturk, Tavares et al., Prevalence of periodontitis and alveolar bone loss in a patient population at Harvard School of Dental Medicine, BMC Oral Health
Maria, Varese, Dentone, Barisione, Bassetti, High prevalence of olfactory and taste disorder during SARS-CoV-2 infection in outpatients, J Med Virol
Meister, Brüggemann, Todt, Virucidal efficacy of different oral rinses against severe acute respiratory syndrome coronavirus 2, J Infect Dis
Natto, Afeef, Bakhrebah, Can periodontal pockets and caries lesions act as reservoirs for Coronavirus?, Mol Oral Microbiol, doi:10.1111/omi.12362
Natto, Afeef, Khalil, Characteristics of oral manifestations in symptomatic non-hospitalized COVID-19 patients: a cross-sectional study on a sample of the Saudi Population, Int J Gen Med
Natto, Alshaeri, Are saudi healthcare students aware of COVID-19, and do they behave safely during viral outbreaks? Niger, J Clin Pract
Natto, Alshaeri, Characteristics of first cases of coronavirus disease 2019 and the effort to prevent the early spread of COVID-19 in Saudi Arabia, Risk Manag Healthc Policy
Natto, Alshehri, Alghamdi, Infection control practices at the dental clinics in Jeddah, Saudi Arabia, J Multidiscip Healthc
Natto, Dental students' knowledge and attitudes about electronic cigarettes: a cross-sectional study at one Saudi University, J Dent Educ
Natto, Parashis, Steffensen, Ganguly, Finkelman et al., Efficacy of collagen matrix seal and collagen sponge on ridge preservation in combination with bone allograft: a randomized controlled clinical trial, J Clin Periodontol
Ng, Marimuthu, Chia, SARS-CoV-2 infection among travelers returning from Wuhan, China, N Engl J Med
O'donnell, Thomas, Stanton, Potential role of oral rinses targeting the viral lipid envelope in SARS-CoV-2 infection, Function
Odeh, Babkair, Abu-Hammad, Borzangy, Abu-Hammad et al., COVID-19: present and future challenges for dental practice, Int J Environ Res Public Health
Recalcati, Cutaneous manifestations in COVID-19: a first perspective, J Eur Acad Dermatol Venereol
Seneviratne, Balan, Ko, Efficacy of commercial mouthrinses on SARS-CoV-2 viral load in saliva: randomized control trial in Singapore, Infection
Sun, Qie, Liu, Ren, Li et al., Clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single arm meta-analysis, J Med Virol
Supranoto, Slot, Addy, Van Der Weijden, The effect of chlorhexidine dentifrice or gel versus chlorhexidine mouthwash on plaque, gingivitis, bleeding and tooth discoloration: a systematic review, Int J Dent Hyg
Yoon, Yoon, Song, Clinical significance of a high SARS-CoV-2 viral load in the saliva, J Korean Med Sci
DOI record:
{
"DOI": "10.1097/md.0000000000028925",
"ISSN": [
"1536-5964"
],
"URL": "http://dx.doi.org/10.1097/MD.0000000000028925",
"abstract": "<jats:p>Several investigations evaluated the possibility of different types of mouth wash rinse in minimizing the SARS-CoV-2 load. However, results still controversial. The study aim is to assess the short-term efficiency of several over-the-counter mouth rinses and lozenges in minimizing the salivary viral load for SARS-CoV-2 in patients with confirmed COVID-19 in comparison to saline. This is a randomized controlled clinical trial with 4 arms. The recruited cases were randomized using a simple randomization technique and were assigned to chlorhexidine digluconate mouth rinse (CHX mouth rinse), 2 mg of chlorhexidine digluconate lozenges (CHX lozenges), povidone iodine mouth rinse (PVP-I mouth rinse) or saline as a control group. Saliva were collected from all study subjects by passive drool technique at two time points. First, prior to intervention with mouth rinse or the lozenges, the baseline saliva sample was collected. Second saliva samples were collected immediately after the mouth rinse. Real time PCR was conducted and the value threshold cycle (Ct) for each sample was recorded. Majority of the participants had an education level of high school or less (60%), were married (68.3), males (58.3%), and non-smokers (58.5%). No statistically significant differences between groups at the two times test (<jats:italic toggle=\"yes\">P</jats:italic> > .05). However, a significant decrease of salivary viral load in all four groups combined (<jats:italic toggle=\"yes\">P</jats:italic>-value for E genes = .027, and for S genes = .006), and in PVP-I mouth rinse specifically (<jats:italic toggle=\"yes\">P</jats:italic> = .003 and <jats:italic toggle=\"yes\">P</jats:italic> = .045, respectively). Povidone iodine mouth rinse showed a potential influence on the reduction of the viral load on a short-term basis. However, longer-term studies of the effect of these products should be conducted.</jats:p>",
"author": [
{
"ORCID": "http://orcid.org/0000-0003-2723-0255",
"affiliation": [
{
"name": "Department of Dental Public Health, Faculty of Dentistry, King Abdulaziz University, Jeddah, Saudi Arabia"
}
],
"authenticated-orcid": false,
"family": "Natto",
"given": "Zuhair S.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Life Science and Environment Research Institute, King Abdulaziz City for Science and Technology (KACST), Riyadh, Saudi Arabia"
}
],
"family": "Bakhrebah",
"given": "Muhammed A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Study and Research Department, King Fahad General Hospital, Jeddah, Saudi Arabia"
}
],
"family": "Afeef",
"given": "Marwah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Intensive Care Unit, King Fahad General Hospital, Jeddah, Saudi Arabia"
}
],
"family": "Al-Harbi",
"given": "Samiah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Life Science and Environment Research Institute, King Abdulaziz City for Science and Technology (KACST), Riyadh, Saudi Arabia"
}
],
"family": "Nassar",
"given": "Majed S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "King Khalid University Hospital, Riyadh, Saudi Arabia"
},
{
"name": "Department of Pathology, King Saud University, Riyadh, Saudi Arabia."
}
],
"family": "Alhetheel",
"given": "Abdulkarim F.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Dental Public Health, Faculty of Dentistry, King Abdulaziz University, Jeddah, Saudi Arabia"
}
],
"family": "Ashi",
"given": "Heba",
"sequence": "additional"
}
],
"container-title": "Medicine",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
8,
10
]
],
"date-time": "2022-08-10T22:06:38Z",
"timestamp": 1660169198000
},
"deposited": {
"date-parts": [
[
2023,
9,
28
]
],
"date-time": "2023-09-28T03:29:29Z",
"timestamp": 1695871769000
},
"indexed": {
"date-parts": [
[
2024,
3,
4
]
],
"date-time": "2024-03-04T16:07:01Z",
"timestamp": 1709568421710
},
"is-referenced-by-count": 11,
"issue": "30",
"issued": {
"date-parts": [
[
2022,
7,
29
]
]
},
"journal-issue": {
"issue": "30",
"published-online": {
"date-parts": [
[
2022
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
7,
29
]
],
"date-time": "2022-07-29T00:00:00Z",
"timestamp": 1659052800000
}
}
],
"link": [
{
"URL": "https://journals.lww.com/10.1097/MD.0000000000028925",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "276",
"original-title": [],
"page": "e28925",
"prefix": "10.1097",
"published": {
"date-parts": [
[
2022,
7,
29
]
]
},
"published-online": {
"date-parts": [
[
2022,
7,
29
]
]
},
"publisher": "Ovid Technologies (Wolters Kluwer Health)",
"reference": [
{
"DOI": "10.1056/NEJMc2003100",
"article-title": "SARS-CoV-2 infection among travelers returning from Wuhan, China.",
"author": "Ng",
"doi-asserted-by": "crossref",
"first-page": "1476",
"journal-title": "N Engl J Med",
"key": "R1-20230928",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1002/jmv.25995",
"article-title": "High prevalence of olfactory and taste disorder during SARS-CoV-2 infection in outpatients.",
"author": "De Maria",
"doi-asserted-by": "crossref",
"first-page": "2310",
"journal-title": "J Med Virol",
"key": "R2-20230928",
"volume": "92",
"year": "2020"
},
{
"article-title": "Cutaneous manifestations in COVID-19: a first perspective.",
"author": "Recalcati",
"first-page": "e212",
"journal-title": "J Eur Acad Dermatol Venereol",
"key": "R3-20230928",
"volume": "34",
"year": "2020"
},
{
"DOI": "10.3390/ijerph17093151",
"article-title": "COVID-19: present and future challenges for dental practice.",
"author": "Odeh",
"doi-asserted-by": "crossref",
"first-page": "3151",
"journal-title": "Int J Environ Res Public Health",
"key": "R4-20230928",
"volume": "17",
"year": "2020"
},
{
"DOI": "10.1177/0194599820934380",
"article-title": "Olfactory and oral manifestations of COVID-19: sex-related symptoms-a potential pathway to early diagnosis.",
"author": "Biadsee",
"doi-asserted-by": "crossref",
"first-page": "722",
"journal-title": "Otolaryngol Head Neck Surg",
"key": "R5-20230928",
"volume": "163",
"year": "2020"
},
{
"DOI": "10.1002/jmv.25735",
"article-title": "Clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single arm meta-analysis.",
"author": "Sun",
"doi-asserted-by": "crossref",
"first-page": "612",
"journal-title": "J Med Virol",
"key": "R6-20230928",
"volume": "92",
"year": "2020"
},
{
"DOI": "10.1186/s12879-021-05950-x",
"article-title": "Serial interval and incubation period of COVID-19: a systematic review and meta-analysis.",
"author": "Alene",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "BMC Infect Dis",
"key": "R8-20230928",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.3346/jkms.2020.35.e195",
"article-title": "Clinical significance of a high SARS-CoV-2 viral load in the saliva.",
"author": "Yoon",
"doi-asserted-by": "crossref",
"first-page": "e195",
"journal-title": "J Korean Med Sci",
"key": "R9-20230928",
"volume": "35",
"year": "2020"
},
{
"DOI": "10.1016/j.joen.2020.03.008",
"article-title": "Coronavirus disease 19 (COVID-19): implications for clinical dental care.",
"author": "Ather",
"doi-asserted-by": "crossref",
"first-page": "584",
"journal-title": "J Endod",
"key": "R10-20230928",
"volume": "46",
"year": "2020"
},
{
"DOI": "10.1177/00220345900690030901",
"article-title": "In vitro virucidal effectiveness of a 0.12%-chlorhexidine gluconate mouthrinse.",
"author": "Bernstein",
"doi-asserted-by": "crossref",
"first-page": "874",
"journal-title": "J Dent Res",
"key": "R11-20230928",
"volume": "69",
"year": "1990"
},
{
"DOI": "10.1034/j.1600-051x.2001.028007610.x",
"article-title": "In vitro effect of oral antiseptics on human immunodeficiency virus-1 and herpes simplex virus type 1.",
"author": "Baqui",
"doi-asserted-by": "crossref",
"first-page": "610",
"journal-title": "J Clin Periodontol",
"key": "R12-20230928",
"volume": "28",
"year": "2001"
},
{
"DOI": "10.1016/j.jdsr.2021.03.001",
"article-title": "Can povidone iodine gargle/mouthrinse inactivate SARS-CoV-2 and decrease the risk of nosocomial and community transmission during the COVID-19 pandemic? An evidence-based update.",
"author": "Chopra",
"doi-asserted-by": "crossref",
"first-page": "39",
"journal-title": "Jpn Dent Sci Rev",
"key": "R13-20230928",
"volume": "57",
"year": "2021"
},
{
"DOI": "10.1016/j.heliyon.2021.e07346",
"article-title": "Salivary SARS-CoV-2 load reduction with mouthwash use: a randomized pilot clinical trial.",
"author": "Eduardo",
"doi-asserted-by": "crossref",
"first-page": "e07346",
"journal-title": "Heliyon",
"key": "R14-20230928",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.1093/infdis/jiaa471",
"article-title": "Virucidal efficacy of different oral rinses against severe acute respiratory syndrome coronavirus 2.",
"author": "Meister",
"doi-asserted-by": "crossref",
"first-page": "1289",
"journal-title": "J Infect Dis",
"key": "R15-20230928",
"volume": "222",
"year": "2020"
},
{
"DOI": "10.1007/s15010-020-01563-9",
"article-title": "Efficacy of commercial mouth-rinses on SARS-CoV-2 viral load in saliva: randomized control trial in Singapore.",
"author": "Seneviratne",
"doi-asserted-by": "crossref",
"first-page": "305",
"journal-title": "Infection",
"key": "R16-20230928",
"volume": "49",
"year": "2021"
},
{
"DOI": "10.1093/function/zqaa002",
"article-title": "Potential role of oral rinses targeting the viral lipid envelope in SARS-CoV-2 infection.",
"author": "O’Donnell",
"doi-asserted-by": "crossref",
"first-page": "zqaa002",
"journal-title": "Function (Oxf)",
"key": "R17-20230928",
"volume": "1",
"year": "2020"
},
{
"DOI": "10.1111/idh.12078",
"article-title": "The effect of chlorhexidine dentifrice or gel versus chlorhexidine mouthwash on plaque, gingivitis, bleeding and tooth discoloration: a systematic review.",
"author": "Supranoto",
"doi-asserted-by": "crossref",
"first-page": "83",
"journal-title": "Int J Dent Hyg",
"key": "R18-20230928",
"volume": "13",
"year": "2015"
},
{
"DOI": "10.1007/s40121-019-00260-x",
"article-title": "Infectious disease management and control with povidone iodine.",
"author": "Eggers",
"doi-asserted-by": "crossref",
"first-page": "581",
"journal-title": "Infect Dis Ther",
"key": "R19-20230928",
"volume": "8",
"year": "2019"
},
{
"DOI": "10.1111/jopr.13209",
"article-title": "Rapid in-vitro inactivation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) using povidone-iodine oral antiseptic rinse.",
"author": "Bidra",
"doi-asserted-by": "crossref",
"first-page": "529",
"journal-title": "J Prosthodont",
"key": "R20-20230928",
"volume": "29",
"year": "2020"
},
{
"DOI": "10.1038/s41415-020-1589-4",
"article-title": "Povidone iodine.",
"author": "Challacombe",
"doi-asserted-by": "crossref",
"first-page": "656",
"journal-title": "Br Dent J",
"key": "R21-20230928",
"volume": "228",
"year": "2020"
},
{
"DOI": "10.1002/JPER.18-0183",
"article-title": "Association between time since quitting smoking and periodontitis in former smokers in the National Health and Nutrition Examination Surveys (NHANES) 2009 to 2012.",
"author": "ALHarthi",
"doi-asserted-by": "crossref",
"first-page": "16",
"journal-title": "J Periodontol",
"key": "R22-20230928",
"volume": "90",
"year": "2012"
},
{
"article-title": "A contemporary look at COVID-19 medications: available and potentially effective drugs.",
"author": "Alshaeri",
"first-page": "9188",
"journal-title": "Eur Rev Med Pharmacol Sci",
"key": "R23-20230928",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1016/j.jebdp.2021.101584",
"article-title": "In vivo evaluation of the virucidal efficacy of chlorhexidine and povidone-iodine mouthwashes against salivary SARS-CoV-2. A randomized-controlled clinical trial.",
"author": "Elzein",
"doi-asserted-by": "crossref",
"first-page": "101584",
"journal-title": "J Evid Based Dent Pract",
"key": "R24-20230928",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1186/s12903-019-0955-6",
"article-title": "Annual alveolar bone loss in older adults taking oral bisphosphonate: a retrospective cohort study.",
"author": "Helmi",
"doi-asserted-by": "crossref",
"first-page": "260",
"journal-title": "BMC Oral Health",
"key": "R25-20230928",
"volume": "19",
"year": "2019"
},
{
"DOI": "10.1186/s12903-020-1015-y",
"article-title": "Annual alveolar bone loss in subjects with cardiovascular disease adjusting for associated systemic diseases and risk factors: a retrospective study.",
"author": "Helmi",
"doi-asserted-by": "crossref",
"first-page": "28",
"journal-title": "BMC Oral Health",
"key": "R26-20230928",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1186/s12903-019-0925-z",
"article-title": "Prevalence of periodontitis and alveolar bone loss in a patient population at Harvard School of Dental Medicine.",
"author": "Helmi",
"doi-asserted-by": "crossref",
"first-page": "254",
"journal-title": "BMC Oral Health",
"key": "R27-20230928",
"volume": "19",
"year": "2019"
},
{
"DOI": "10.21815/JDE.019.162",
"article-title": "Dental students’ knowledge and attitudes about electronic cigarettes: a cross-sectional study at one Saudi University.",
"author": "Natto",
"doi-asserted-by": "crossref",
"first-page": "27",
"journal-title": "J Dent Educ",
"key": "R28-20230928",
"volume": "84",
"year": "2020"
},
{
"DOI": "10.1111/omi.12362",
"article-title": "Can periodontal pockets and caries lesions act as reservoirs for Coronavirus?",
"author": "Natto",
"doi-asserted-by": "crossref",
"journal-title": "Mol Oral Microbiol",
"key": "R29-20230928",
"year": "2022"
},
{
"DOI": "10.2147/IJGM.S331611",
"article-title": "Characteristics of oral manifestations in symptomatic non-hospitalized COVID-19 patients: a cross-sectional study on a sample of the Saudi Population.",
"author": "Natto",
"doi-asserted-by": "crossref",
"first-page": "9547",
"journal-title": "Int J Gen Med",
"key": "R30-20230928",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.4103/njcp.njcp_259_20",
"article-title": "Are saudi healthcare students aware of COVID-19, and do they behave safely during viral outbreaks?",
"author": "Natto",
"doi-asserted-by": "crossref",
"first-page": "406",
"journal-title": "Niger J Clin Pract",
"key": "R31-20230928",
"volume": "24",
"year": "2021"
},
{
"DOI": "10.2147/RMHP.S278394",
"article-title": "Characteristics of first cases of coronavirus disease 2019 and the effort to prevent the early spread of COVID-19 in Saudi Arabia.",
"author": "Natto",
"doi-asserted-by": "crossref",
"first-page": "315",
"journal-title": "Risk Manag Healthc Policy",
"key": "R32-20230928",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.2147/JMDH.S330567",
"article-title": "Infection control practices at the dental clinics in Jeddah, Saudi Arabia.",
"author": "Natto",
"doi-asserted-by": "crossref",
"first-page": "2951",
"journal-title": "J Multidiscip Healthc",
"key": "R33-20230928",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.1111/jcpe.12722",
"article-title": "Efficacy of collagen matrix seal and collagen sponge on ridge preservation in combination with bone allograft: a randomized controlled clinical trial.",
"author": "Natto",
"doi-asserted-by": "crossref",
"first-page": "649",
"journal-title": "J Clin Periodontol",
"key": "R34-20230928",
"volume": "44",
"year": "2017"
}
],
"reference-count": 33,
"references-count": 33,
"relation": {},
"resource": {
"primary": {
"URL": "https://journals.lww.com/10.1097/MD.0000000000028925"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "The short-term effect of different chlorhexidine forms versus povidone iodine mouth rinse in minimizing the oral SARS-CoV-2 viral load: An open label randomized controlled clinical trial study",
"type": "journal-article",
"volume": "101"
}
