Value of montelukast as a potential treatment of post-COVID-19 persistent cough: a non-randomized controlled pilot study
et al., The Egyptian Journal of Bronchology, doi:10.1186/s43168-022-00154-6, Sep 2022
32nd treatment shown to reduce risk in
November 2021, now with p = 0.0041 from 9 studies.
Lower risk for hospitalization and cases.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
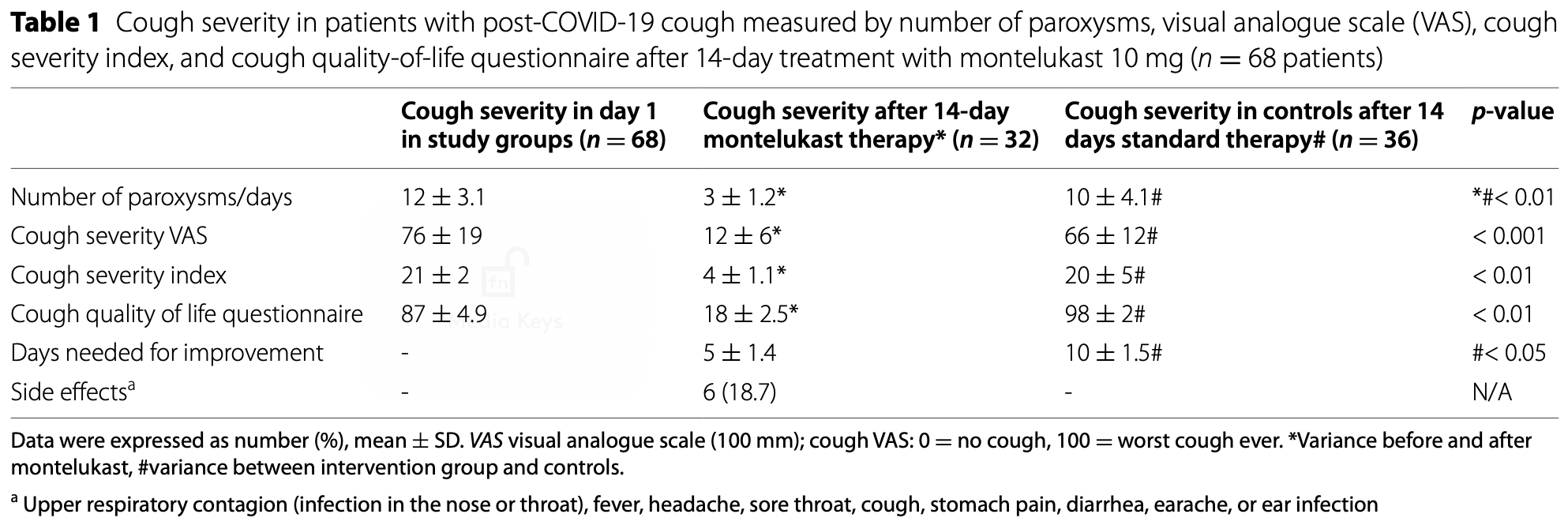
RCT 68 post-COVID-19 outpatients showing improvement in cough severity measures with montelukast treatment. The montelukast group had a greater reduction in number of cough paroxysms per day, cough severity visual analog scale, cough severity index, and improved cough quality of life scores compared to the control group. The montelukast group also had a shorter duration of cough.
|
improvement, 50.0% lower, relative time 0.50, p < 0.001, treatment mean 5.0 (±1.4) n=32, control mean 10.0 (±1.5) n=36.
|
|
paroxysms/day, 70.0% lower, relative time 0.30, p < 0.001, treatment mean 3.0 (±1.2) n=32, control mean 10.0 (±4.1) n=36.
|
|
VAS, 81.8% lower, relative time 0.18, p < 0.001, treatment mean 12.0 (±6.0) n=32, control mean 66.0 (±12.0) n=36.
|
|
severity index, 80.0% lower, relative time 0.20, p < 0.001, treatment mean 4.0 (±1.1) n=32, control mean 20.0 (±5.0) n=36.
|
|
QOL, 81.6% lower, relative time 0.18, p < 0.001, treatment mean 18.0 (±2.5) n=32, control mean 98.0 (±2.0) n=36.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Mohamed Hussein et al., 15 Sep 2022, Randomized Controlled Trial, Egypt, peer-reviewed, mean age 43.0, 7 authors, post-COVID cough.
Contact: islamgalal76@yahoo.com (corresponding author).
Value of montelukast as a potential treatment of post-COVID-19 persistent cough: a non-randomized controlled pilot study
The Egyptian Journal of Bronchology, doi:10.1186/s43168-022-00154-6
Background: This pilot study included 68 cases with post-COVID-19 persistent cough (> 8 weeks), randomly allocated into two groups; intervention group (32 patients) received standard cough therapy, and montelukast 10 mg/ day for 14 days and control group (36 patients) received only cough sedatives.
Results: We found a significant improvement in the number of cough paroxysms/day, cough severity visual analog scale, cough severity index and cough quality of life, shorter duration improvement, and minimal side effects in the interventional group.
Conclusions: We suggest that montelukast may be effective to reduce the duration and severity of the persistent post-COVID-19 cough and further improve quality of life.
Abbreviations VAS: Cough visual analog scale; RSV: Respiratory syncytial virus; ACE: Angiotensin-converting enzyme.
Authors' contributions IG was the principal investigator, formulated the idea, and wrote the first draft of discussion. AMH, MEI, and HM collected the data, formulated the results, and edited the final draft and revision. NM was responsible for methodology and statistical analysis. HA and KK were responsible for data acquisition, review search, and writing the primary draft. The authors read and approved the final manuscript.
Declarations Ethics approval and consent to participate The Research Ethics Committee at the Faculty of Medicine, Aswan University, has approved the study (IRB number: aswu/469/7/2020), and all patients provided written informed consent before participation.
Consent for publication The manuscript has been read and approved by all the authors.
Competing interests The authors declare that they have no competing interests.
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Assaf, Davis, Mccorkell, An analysis of the prolonged COVID-19 symptoms survey by patient-led research team, Patient Led Res
Barré, Sabatier, Annweiler, Montelukast drug may improve COVID-19 prognosis: a review of evidence, Front Pharmacol
Bisgaard, Flores-Nunez, Goh, Azimi, Halkas et al., Study of montelukast for the treatment of respiratory symptoms of post-respiratory syncytial virus bronchiolitis in children, Am J Respir Crit Care Med
Bozek, Winterstein, Montelukast's ability to fight COVID-19 infection, J Asthma, doi:10.1080/02770903.2020.1786112
Carfì, Bernabei, Landi, For the gemelli against COVID-19 post-acute care study group. Persistent symptoms in patients after acute COVID-19, JAMA, doi:10.1001/jama.2020.12603
Chen, Li, Wang, Zou, Montelukast, an anti-asthmatic drug, inhibits Zika virus infection by disrupting viral integrity, Front Microbiol
Fidan, Aydoğdu, As a potential treatment of COVID-19: montelukast, Med Hypotheses, doi:10.1016/j.mehy.2020.109828
French, Irwin, Fletcher, Evaluation of a cough-specific quality-of-life questionnaire, Chest
Galal, Hussein, Amin, Saad, Zayan et al., Determinants of persistent post-COVID-19 symptoms: value of a novel COVID-19 symptom score, Egypt J Bronchol
Huynh, Wang, Luan, In silico exploration of the molecular mechanism of clinically oriented drugs for possibly inhibiting SARS-CoV-2's main protease, J Phys Chem Lett
Peroni, Pescollderungg, Sandri, Chinellato, Boner et al., Time-effect of montelukast on protection against exerciseinduced bronchoconstriction, Respir Med
Ponsioen, Hop, Vermue, Dekhuijzen, Bohnen, Efficacy of fluticasone on cough: a randomised controlled trial, Eur Respir J
Ruiz, Nevers, Hernández, Ahnou, Brillet et al., & Ahmed-Belkacem A (2020) MK-571, a cysteinyl leukotriene receptor 1 antagonist, inhibits hepatitis C virus replication, Antimicrob Agents Chemother
Shembel, Rosen, Zullo, Gartner-Schmidt, Development and validation of the cough severity index: a severity index for chronic cough related to the upper airway, Laryngoscope, doi:10.1002/lary.23916
Spector, Tan, Effectiveness of montelukast in the treatment of cough variant asthma, Ann Allergy Asthma Immunol
Wang, Birring, Taylor, Fry, Hay et al., Montelukast for postinfectious Mohamed Hussein et al. The Egyptian Journal of Bronchology (2022) 16:52 cough in adults: a double-blind randomised placebo-controlled trial. The lancet, Respir Med
Woodcock, Mcleod, Sadeh, Smith, The efficacy of a NOP1 agonist (SCH486757) in subacute cough, Lung
Zhu, Kuang, Deng, Clinical analysis of montelukast in the treatment of post-infectious cough, China Pharmacy
DOI record:
{
"DOI": "10.1186/s43168-022-00154-6",
"ISSN": [
"1687-8426",
"2314-8551"
],
"URL": "http://dx.doi.org/10.1186/s43168-022-00154-6",
"abstract": "<jats:title>Abstract</jats:title><jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>This pilot study included 68 cases with post-COVID-19 persistent cough (> 8 weeks), randomly allocated into two groups; intervention group (32 patients) received standard cough therapy, and montelukast 10 mg/day for 14 days and control group (36 patients) received only cough sedatives.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>We found a significant improvement in the number of cough paroxysms/day, cough severity visual analog scale, cough severity index and cough quality of life, shorter duration improvement, and minimal side effects in the interventional group.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>We suggest that montelukast may be effective to reduce the duration and severity of the persistent post-COVID-19 cough and further improve quality of life.</jats:p>\n </jats:sec>",
"alternative-id": [
"154"
],
"article-number": "52",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "27 June 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "28 August 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "15 September 2022"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "The Research Ethics Committee at the Faculty of Medicine, Aswan University, has approved the study (IRB number: aswu/469/7/2020), and all patients provided written informed consent before participation."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "The manuscript has been read and approved by all the authors."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "The authors declare that they have no competing interests."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-7111-2195",
"affiliation": [],
"authenticated-orcid": false,
"family": "Mohamed Hussein",
"given": "Aliae A. R.",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0001-6068-5724",
"affiliation": [],
"authenticated-orcid": false,
"family": "Ibrahim",
"given": "Mohamed Eltaher A. A.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6002-6796",
"affiliation": [],
"authenticated-orcid": false,
"family": "Makhlouf",
"given": "Hoda A.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2949-4369",
"affiliation": [],
"authenticated-orcid": false,
"family": "Makhlouf",
"given": "Nahed A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abd-Elaal",
"given": "Howaida K.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kholief",
"given": "Karima M. S.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0844-4215",
"affiliation": [],
"authenticated-orcid": false,
"family": "Sayed",
"given": "Islam G.",
"sequence": "additional"
}
],
"container-title": "The Egyptian Journal of Bronchology",
"container-title-short": "Egypt J Bronchol",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2022,
9,
15
]
],
"date-time": "2022-09-15T12:07:37Z",
"timestamp": 1663243657000
},
"deposited": {
"date-parts": [
[
2022,
9,
15
]
],
"date-time": "2022-09-15T12:11:37Z",
"timestamp": 1663243897000
},
"indexed": {
"date-parts": [
[
2024,
5,
4
]
],
"date-time": "2024-05-04T01:37:09Z",
"timestamp": 1714786629793
},
"is-referenced-by-count": 6,
"issue": "1",
"issued": {
"date-parts": [
[
2022,
9,
15
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2022,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
9,
15
]
],
"date-time": "2022-09-15T00:00:00Z",
"timestamp": 1663200000000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
9,
15
]
],
"date-time": "2022-09-15T00:00:00Z",
"timestamp": 1663200000000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s43168-022-00154-6.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s43168-022-00154-6/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s43168-022-00154-6.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2022,
9,
15
]
]
},
"published-online": {
"date-parts": [
[
2022,
9,
15
]
]
},
"published-print": {
"date-parts": [
[
2022,
12
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1001/jama.2020.12603",
"author": "A Carfì",
"doi-asserted-by": "publisher",
"first-page": "603",
"issue": "6",
"journal-title": "JAMA.",
"key": "154_CR1",
"unstructured": "Carfì A, Bernabei R, Landi F (2020) For the gemelli against COVID-19 post-acute care study group. Persistent symptoms in patients after acute COVID-19. JAMA. 324(6):603–605. https://doi.org/10.1001/jama.2020.12603",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1186/s43168-020-00049-4",
"doi-asserted-by": "crossref",
"key": "154_CR2",
"unstructured": "Galal I, Hussein AA, Amin MT, Saad MM, Zayan HEE, Abdelsayed M Z, ... & Hashem M. K (2021) Determinants of persistent post-COVID-19 symptoms: value of a novel COVID-19 symptom score. Egypt J Bronchol 15(1):1–8"
},
{
"DOI": "10.1021/acs.jpclett.0c00994",
"author": "T Huynh",
"doi-asserted-by": "publisher",
"first-page": "4413",
"issue": "11",
"journal-title": "J Phys Chem Lett",
"key": "154_CR3",
"unstructured": "Huynh T, Wang H, Luan B (2020) In silico exploration of the molecular mechanism of clinically oriented drugs for possibly inhibiting SARS-CoV-2’s main protease. J Phys Chem Lett 11(11):4413–4420",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1016/S1081-1206(10)61493-7",
"author": "SL Spector",
"doi-asserted-by": "publisher",
"first-page": "232",
"issue": "3",
"journal-title": "Ann Allergy Asthma Immunol",
"key": "154_CR4",
"unstructured": "Spector SL, Tan RA (2004) Effectiveness of montelukast in the treatment of cough variant asthma. Ann Allergy Asthma Immunol 93(3):232–236",
"volume": "93",
"year": "2004"
},
{
"DOI": "10.1016/j.rmed.2011.08.007",
"author": "DG Peroni",
"doi-asserted-by": "publisher",
"first-page": "1790",
"issue": "12",
"journal-title": "Respir Med",
"key": "154_CR5",
"unstructured": "Peroni DG, Pescollderungg L, Sandri M, Chinellato I, Boner AL, Piacentini GL (2011) Time-effect of montelukast on protection against exercise-induced bronchoconstriction. Respir Med 105(12):1790–1797",
"volume": "105",
"year": "2011"
},
{
"author": "LQ Zhu",
"first-page": "2067",
"journal-title": "China Pharmacy",
"key": "154_CR6",
"unstructured": "Zhu LQ, Kuang JL, Deng ZC (2011) Clinical analysis of montelukast in the treatment of post-infectious cough. China Pharmacy 22:2067–2069",
"volume": "22",
"year": "2011"
},
{
"DOI": "10.1164/rccm.200706-910OC",
"author": "H Bisgaard",
"doi-asserted-by": "publisher",
"first-page": "854",
"issue": "8",
"journal-title": "Am J Respir Crit Care Med",
"key": "154_CR7",
"unstructured": "Bisgaard H, Flores-Nunez A, Goh A, Azimi P, Halkas A, Malice MP, Marchal JL, Dass SB, Reiss TF, Knorr BA (2008) Study of montelukast for the treatment of respiratory symptoms of post–respiratory syncytial virus bronchiolitis in children. Am J Respir Crit Care Med 178(8):854–860",
"volume": "178",
"year": "2008"
},
{
"DOI": "10.3389/fphar.2020.01344",
"author": "J Barré",
"doi-asserted-by": "publisher",
"first-page": "1344",
"journal-title": "Front Pharmacol",
"key": "154_CR8",
"unstructured": "Barré J, Sabatier JM, Annweiler C (2020) Montelukast drug may improve COVID-19 prognosis: a review of evidence. Front Pharmacol 11:1344",
"volume": "11",
"year": "2020"
},
{
"key": "154_CR9",
"unstructured": "Assaf G, Davis H, McCorkell L et al (2020) An analysis of the prolonged COVID-19 symptoms survey by patient-led research team. Patient Led Res https://patientresearchcovid19.com/"
},
{
"author": "British Thoracic Society",
"key": "154_CR10",
"unstructured": "British Thoracic Society (2020) British Thoracic Society guidance on respiratory follow up of patients with a clinico-radiological diagnosis of COVID-19 pneumonia https://www.brit-thoracic.org.uk/document-library/quality-improvement/covid-19/resp-follow-up-guidance-post-covid-pneumonia/",
"volume-title": "British Thoracic Society guidance on respiratory follow up of patients with a clinico-radiological diagnosis of COVID-19 pneumonia",
"year": "2020"
},
{
"author": "Homerton University Hospital",
"key": "154_CR11",
"unstructured": "Homerton University Hospital (2020) Post COVID-19 patient information pack https://www.hackneycitizen.co.uk/wp-content/uploads/Post-COVID-19-information-pack-5.pdf",
"volume-title": "Post COVID-19 patient information pack",
"year": "2020"
},
{
"DOI": "10.1002/lary.23916",
"doi-asserted-by": "publisher",
"key": "154_CR12",
"unstructured": "Shembel AC, Rosen CA, Zullo TG, Gartner-Schmidt JL (2013) Development and validation of the cough severity index: a severity index for chronic cough related to the upper airway. Laryngoscope. https://doi.org/10.1002/lary.23916"
},
{
"DOI": "10.1378/chest.121.4.1123",
"author": "C French",
"doi-asserted-by": "publisher",
"first-page": "1123",
"journal-title": "Chest.",
"key": "154_CR13",
"unstructured": "French C, Irwin RS, Fletcher KE et al (2002) Evaluation of a cough-specific quality-of-life questionnaire. Chest. 121:1123–1131",
"volume": "121",
"year": "2002"
},
{
"author": "K Wang",
"first-page": "35",
"issue": "1",
"journal-title": "Respir Med",
"key": "154_CR14",
"unstructured": "Wang K, Birring SS, Taylor K, Fry NK, Hay AD, Moore M, Jin J, Perera R, Farmer A, Little P, Harrison TG (2014) Montelukast for postinfectious cough in adults: a double-blind randomised placebo-controlled trial. The lancet. Respir Med 2(1):35–43",
"volume": "2",
"year": "2014"
},
{
"DOI": "10.1183/09031936.04.00053604",
"author": "BP Ponsioen",
"doi-asserted-by": "publisher",
"first-page": "147",
"issue": "1",
"journal-title": "Eur Respir J",
"key": "154_CR15",
"unstructured": "Ponsioen BP, Hop WC, Vermue NA, Dekhuijzen PN, Bohnen AM (2005) Efficacy of fluticasone on cough: a randomised controlled trial. Eur Respir J 25(1):147–152",
"volume": "25",
"year": "2005"
},
{
"DOI": "10.1007/s00408-009-9197-8",
"author": "A Woodcock",
"doi-asserted-by": "publisher",
"first-page": "47",
"issue": "1",
"journal-title": "Lung.",
"key": "154_CR16",
"unstructured": "Woodcock A, McLeod RL, Sadeh J, Smith JA (2010) The efficacy of a NOP1 agonist (SCH486757) in subacute cough. Lung. 188(1):47–52",
"volume": "188",
"year": "2010"
},
{
"DOI": "10.1080/02770903.2020.1786112",
"doi-asserted-by": "publisher",
"key": "154_CR17",
"unstructured": "Bozek A, Winterstein J (2020) Montelukast’s ability to fight COVID-19 infection. J Asthma 1–2. https://doi.org/10.1080/02770903.2020.1786112"
},
{
"DOI": "10.1016/j.mehy.2020.109828",
"author": "C Fidan",
"doi-asserted-by": "publisher",
"first-page": "109828",
"journal-title": "Med Hypotheses",
"key": "154_CR18",
"unstructured": "Fidan C, Aydoğdu A (2020) As a potential treatment of COVID-19: montelukast. Med Hypotheses 142:109828. https://doi.org/10.1016/j.mehy.2020.109828 Epub 2020 May 11. PMID: 32416408; PMCID: PMC7211747",
"volume": "142",
"year": "2020"
},
{
"DOI": "10.3389/fmicb.2019.03079",
"author": "Y Chen",
"doi-asserted-by": "publisher",
"first-page": "3079",
"journal-title": "Front Microbiol",
"key": "154_CR19",
"unstructured": "Chen Y, Li Y, Wang X, Zou P (2020) Montelukast, an anti-asthmatic drug, inhibits Zika virus infection by disrupting viral integrity. Front Microbiol 10:3079",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1128/AAC.02078-19",
"doi-asserted-by": "crossref",
"key": "154_CR20",
"unstructured": "Ruiz I, Nevers Q, Hernández E, Ahnou N, Brillet R, Softic L, ... & Ahmed-Belkacem A (2020) MK-571, a cysteinyl leukotriene receptor 1 antagonist, inhibits hepatitis C virus replication. Antimicrob Agents Chemother 64(6):e02078–19"
}
],
"reference-count": 20,
"references-count": 20,
"relation": {},
"resource": {
"primary": {
"URL": "https://ejb.springeropen.com/articles/10.1186/s43168-022-00154-6"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Value of montelukast as a potential treatment of post-COVID-19 persistent cough: a non-randomized controlled pilot study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "16"
}
