Clinical efficacy of casirivimab-imdevimab antibody combination treatment in patients with COVID-19 Delta variant
et al., Journal of Infection and Chemotherapy, doi:10.1016/j.jiac.2022.05.012, May 2022
19th treatment shown to reduce risk in
March 2021, now with p = 0.000095 from 34 studies, recognized in 52 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
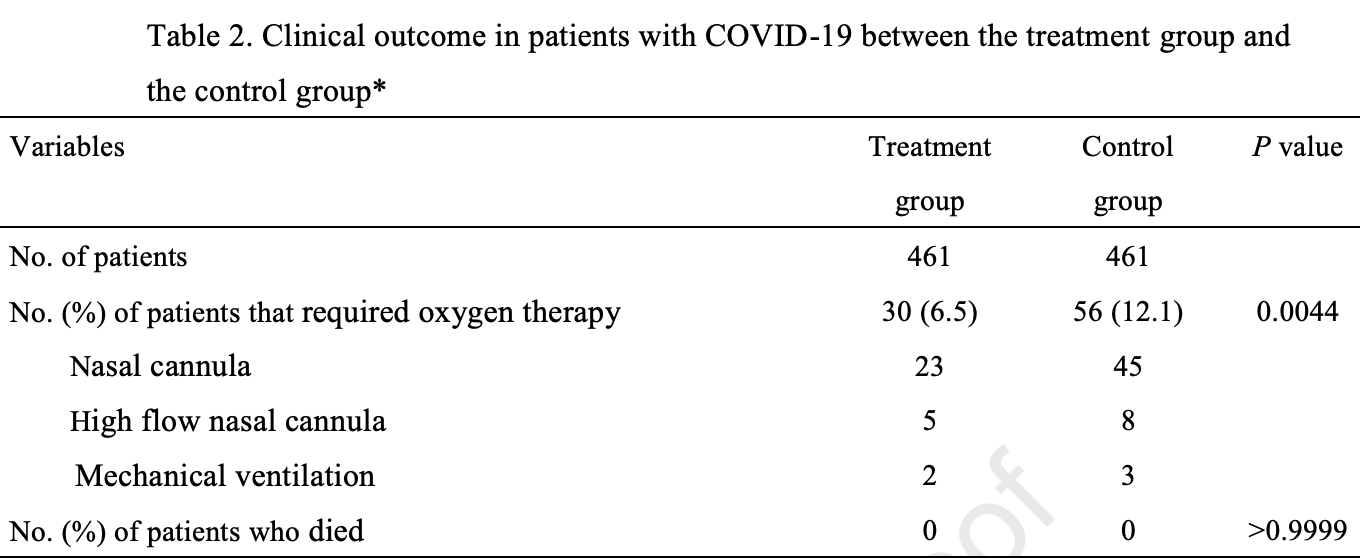
Retrospective 461 patients treated with casirivimab/imdevimab in Japan, and 461 matched controls, showing lower oxygen requirements with treatment.
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for many omicron variants1-7.
Standard of Care (SOC) for COVID-19 in the study country,
Japan, is very poor with very low average efficacy for approved treatments8.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of mechanical ventilation, 33.3% lower, RR 0.67, p = 1.00, treatment 2 of 461 (0.4%), control 3 of 461 (0.7%), NNT 461.
|
|
risk of oxygen therapy, 46.4% lower, RR 0.54, p = 0.004, treatment 30 of 461 (6.5%), control 56 of 461 (12.1%), NNT 18.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
4.
Tatham et al., Lack of Ronapreve (REGN-CoV; casirivimab and imdevimab) virological efficacy against the SARS-CoV 2 Omicron variant (B.1.1.529) in K18-hACE2 mice, bioRxiv, doi:10.1101/2022.01.23.477397.
5.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
6.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
Miyashita et al., 26 May 2022, retrospective, Japan, peer-reviewed, 6 authors, average treatment delay 4.0 days.
Clinical efficacy of casirivimab-imdevimab antibody combination treatment in patients with COVID-19 Delta variant
Journal of Infection and Chemotherapy, doi:10.1016/j.jiac.2022.05.012
This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest The authors declare that they have no conflicts of interest.
Author's contributions: All the authors conceived the study, participated in its design and coordination and collected and managed the data, including quality control. NM and YN drafted the manuscript, and all authors contributed substantially to its revision. All the authors read and approved the final manuscript.
Ethical approval and consent to participate J o u r n a l P r e -p r o o f
References
Baum, Fulton, Wloga, Copin, Pascal et al., Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies, Science
Bierle, Ganesh, Razonable, Breakthrough COVID-19 and casirivimabimdevimab treatment during a SARS-CoV-2 B1.617.2 (Delta) surge, J Clin Virol
Ghosn, Chaimani, Evrenoglou, Davidson, Graña et al., Interleukin-6 blocking agents for treating COVID-19: a living systematic review, Cochrane Database Syst Rev
Hansen, Baum, Pascal, Russo, Giordano et al., Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail, Science
Marconi, Ramanan, De Bono, Kartman, Krishnan et al., Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebocontrolled phase 3 trial, Lancet Respir Med
O'brien, Forleo-Neto, Musser, Chan, Sarkar, Subcutaneous REGEN-COV antibody combination to prevent Covid-19, N Engl J Med
O'brien, Forleo-Neto, Sarkar, Isa, Hou et al., Effect of subcutaneous casirivimab and imdevimab antibody combination vs placebo on J o u r n a l P r e -p r o o f development of symptomatic COVID-19 in early asymptomatic SARS-CoV-2 infection. A Randomized Clinical Trial, JAMA
Planas, Saunders, Maes, Guivel-Benhassine, Planchais et al., Considerable escape of SARS-CoV-2 Omicron to antibody neutralization, Nature
Siemieniuk, Bartoszko, Ge, Zeraatkar, Izcovich et al., Drug treatments for covid-19: living systematic review and network meta-analysis, BMJ
Takashita, Kinoshita, Yamayoshi, Sakai-Tagawa, Fujisaki et al., Efficacy of Antibodies and Antiviral Drugs against Covid-19 Omicron Variant, N Engl J Med
Takashita, Kinoshita, Yamayoshi, Sakai-Tagawa, Fujisaki et al., Efficacy of antiviral agents against the SARS-CoV-2 Omicron subvariant BA.2, N Engl J Med Mar, doi:10.1056/NEJMc2201933
Wagner, Griesel, Mikolajewska, Mueller, Nothacker et al., Systemic corticosteroids for the treatment of COVID-19, Cochrane Database Syst Rev
Weinreich, Sivapalasingam, Norton, Ali, Gao et al., REGEN-COV antibody combination and outcomes in outpatients with Covid-19, N Engl J Med
Weinreich, Sivapalasingam, Norton, Ali, Gao et al., REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19, N Engl J Med
Zhu, Zhang, Li, Yang, Song, A novel coronavirus from patients with pneumonia in China, 2019, N Engl J Med
DOI record:
{
"DOI": "10.1016/j.jiac.2022.05.012",
"ISSN": [
"1341-321X"
],
"URL": "http://dx.doi.org/10.1016/j.jiac.2022.05.012",
"alternative-id": [
"S1341321X22001611"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Clinical efficacy of casirivimab-imdevimab antibody combination treatment in patients with COVID-19 Delta variant"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Journal of Infection and Chemotherapy"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.jiac.2022.05.012"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2022 Japanese Society of Chemotherapy and The Japanese Association for Infectious Diseases. Published by Elsevier Ltd. All rights reserved."
}
],
"author": [
{
"affiliation": [],
"family": "Miyashita",
"given": "Naoyuki",
"sequence": "first"
},
{
"affiliation": [],
"family": "Nakamori",
"given": "Yasushi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ogata",
"given": "Makoto",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fukuda",
"given": "Naoki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yamura",
"given": "Akihisa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ishiura",
"given": "Yoshihisa",
"sequence": "additional"
}
],
"container-title": "Journal of Infection and Chemotherapy",
"container-title-short": "Journal of Infection and Chemotherapy",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.es",
"clinicalkey.com.au",
"jiac-j.com",
"clinicalkey.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2022,
5,
26
]
],
"date-time": "2022-05-26T06:30:56Z",
"timestamp": 1653546656000
},
"deposited": {
"date-parts": [
[
2022,
7,
10
]
],
"date-time": "2022-07-10T10:26:54Z",
"timestamp": 1657448814000
},
"indexed": {
"date-parts": [
[
2023,
5,
24
]
],
"date-time": "2023-05-24T10:51:16Z",
"timestamp": 1684925476889
},
"is-referenced-by-count": 2,
"issue": "9",
"issued": {
"date-parts": [
[
2022,
9
]
]
},
"journal-issue": {
"issue": "9",
"published-print": {
"date-parts": [
[
2022,
9
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
9,
1
]
],
"date-time": "2022-09-01T00:00:00Z",
"timestamp": 1661990400000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1341321X22001611?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1341321X22001611?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "1344-1346",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
9
]
]
},
"published-print": {
"date-parts": [
[
2022,
9
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1056/NEJMoa2001017",
"article-title": "A novel coronavirus from patients with pneumonia in China, 2019",
"author": "Zhu",
"doi-asserted-by": "crossref",
"first-page": "727",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jiac.2022.05.012_bib1",
"volume": "382",
"year": "2020"
},
{
"article-title": "Systemic corticosteroids for the treatment of COVID-19",
"author": "Wagner",
"first-page": "CD014963",
"journal-title": "Cochrane Database Syst Rev",
"key": "10.1016/j.jiac.2022.05.012_bib2",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(21)00331-3",
"article-title": "Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial",
"author": "Marconi",
"doi-asserted-by": "crossref",
"first-page": "1407",
"journal-title": "Lancet Respir Med",
"key": "10.1016/j.jiac.2022.05.012_bib3",
"volume": "9",
"year": "2021"
},
{
"article-title": "Interleukin-6 blocking agents for treating COVID-19: a living systematic review",
"author": "Ghosn",
"first-page": "CD013881",
"journal-title": "Cochrane Database Syst Rev",
"key": "10.1016/j.jiac.2022.05.012_bib4",
"volume": "3",
"year": "2021"
},
{
"DOI": "10.1136/bmj.m2980",
"article-title": "Drug treatments for covid-19: living systematic review and network meta-analysis",
"author": "Siemieniuk",
"doi-asserted-by": "crossref",
"first-page": "m2980",
"journal-title": "BMJ",
"key": "10.1016/j.jiac.2022.05.012_bib5",
"volume": "370",
"year": "2020"
},
{
"DOI": "10.1126/science.abd0831",
"article-title": "Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies",
"author": "Baum",
"doi-asserted-by": "crossref",
"journal-title": "Science",
"key": "10.1016/j.jiac.2022.05.012_bib6",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1126/science.abd0827",
"article-title": "Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail",
"author": "Hansen",
"doi-asserted-by": "crossref",
"journal-title": "Science",
"key": "10.1016/j.jiac.2022.05.012_bib7",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2035002",
"article-title": "REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19",
"author": "Weinreich",
"doi-asserted-by": "crossref",
"first-page": "238",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jiac.2022.05.012_bib8",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2108163",
"article-title": "REGEN-COV antibody combination and outcomes in outpatients with Covid-19",
"author": "Weinreich",
"doi-asserted-by": "crossref",
"first-page": "e81",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jiac.2022.05.012_bib9",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2109682",
"article-title": "Subcutaneous REGEN-COV antibody combination to prevent Covid-19",
"author": "O'Brien",
"doi-asserted-by": "crossref",
"first-page": "1184",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jiac.2022.05.012_bib10",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.24939",
"article-title": "Effect of subcutaneous casirivimab and imdevimab antibody combination vs placebo on development of symptomatic COVID-19 in early asymptomatic SARS-CoV-2 infection. A Randomized Clinical Trial",
"author": "O'Brien",
"doi-asserted-by": "crossref",
"first-page": "432",
"journal-title": "JAMA",
"key": "10.1016/j.jiac.2022.05.012_bib11",
"volume": "327",
"year": "2022"
},
{
"DOI": "10.1016/j.jcv.2021.105026",
"article-title": "Breakthrough COVID-19 and casirivimab-imdevimab treatment during a SARS-CoV-2 B1.617.2 (Delta) surge",
"author": "Bierle",
"doi-asserted-by": "crossref",
"journal-title": "J Clin Virol",
"key": "10.1016/j.jiac.2022.05.012_bib12",
"volume": "145",
"year": "2021"
},
{
"DOI": "10.1038/s41586-021-04389-z",
"article-title": "Considerable escape of SARS-CoV-2 Omicron to antibody neutralization",
"author": "Planas",
"doi-asserted-by": "crossref",
"first-page": "671",
"journal-title": "Nature",
"key": "10.1016/j.jiac.2022.05.012_bib13",
"volume": "602",
"year": "2022"
},
{
"DOI": "10.1056/NEJMc2119407",
"article-title": "Efficacy of antibodies and antiviral drugs against covid-19 Omicron variant",
"author": "Takashita",
"doi-asserted-by": "crossref",
"first-page": "995",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jiac.2022.05.012_bib14",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMc2201933",
"doi-asserted-by": "crossref",
"key": "10.1016/j.jiac.2022.05.012_bib15",
"unstructured": "Takashita E, Kinoshita N, Yamayoshi S, Sakai-Tagawa Y, Fujisaki S, Ito M, et al. Efficacy of antiviral agents against the SARS-CoV-2 Omicron subvariant BA.2. N Engl J Med Mar 9;NEJMc2201933. doi: 10.1056/NEJMc2201933."
}
],
"reference-count": 15,
"references-count": 15,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1341321X22001611"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Pharmacology (medical)",
"Microbiology (medical)"
],
"subtitle": [],
"title": "Clinical efficacy of casirivimab-imdevimab antibody combination treatment in patients with COVID-19 Delta variant",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "28"
}
