Resveratrol and Copper for treatment of severe COVID-19: an observational study (RESCU 002)
et al., medRxiv, doi:10.1101/2020.07.21.20151423, RESCU 002, CTRI/2020/06/026256, Jul 2020
Retrospective 230 severe COVID-19 patients in India, showing lower mortality with resveratrol + copper, without statistical significance. This study followed preclinical data showing that sepsis-related cytokine storm and fatality in mice could be prevented with oral administration of resveratrol and copper.
|
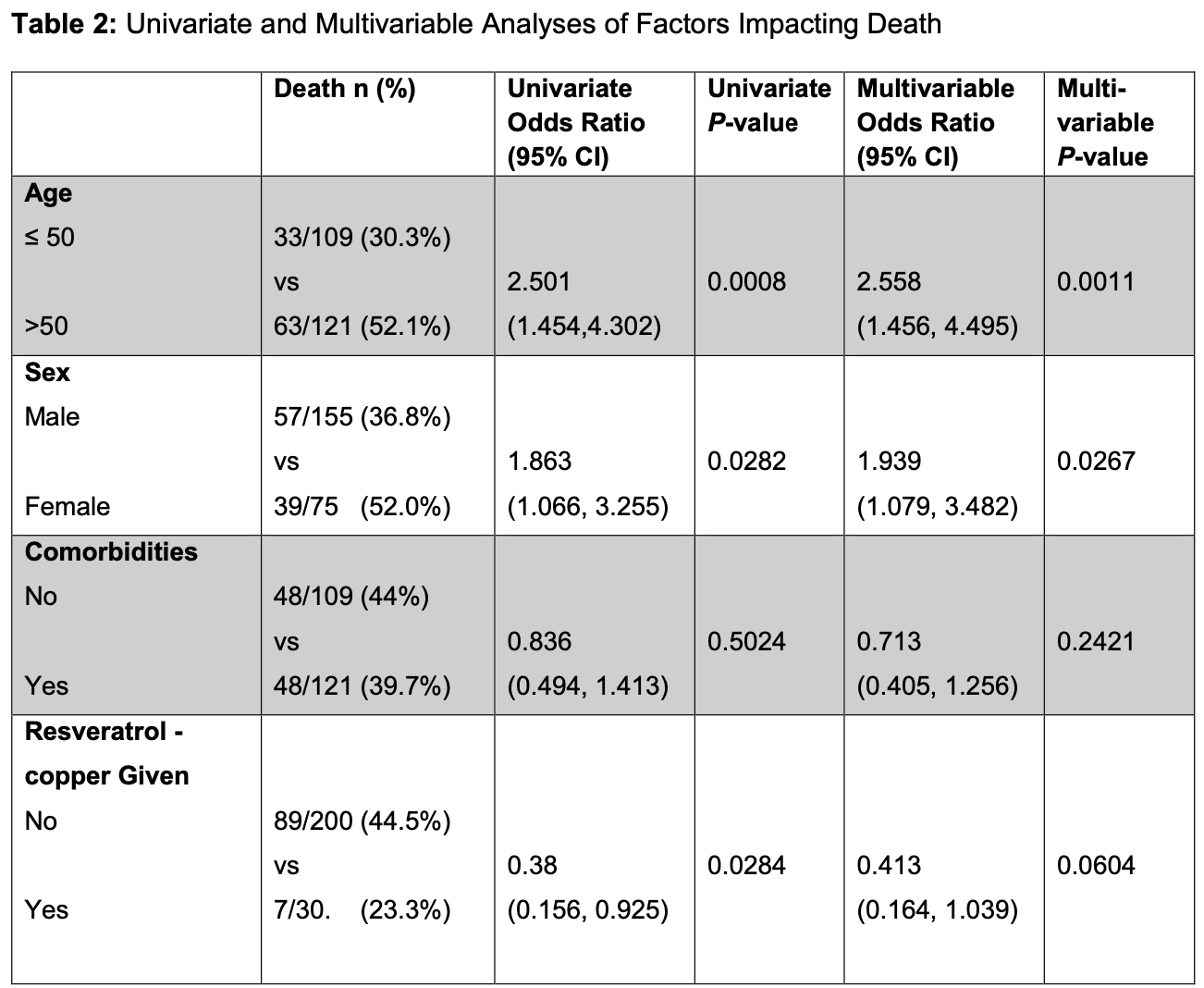
risk of death, 44.1% lower, RR 0.56, p = 0.06, treatment 7 of 30 (23.3%), control 89 of 200 (44.5%), NNT 4.7, adjusted per study, odds ratio converted to relative risk, multivariable.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Mittra et al., 29 Jul 2020, retrospective, India, preprint, 12 authors, study period 1 April, 2020 - 13 May, 2020, this trial uses multiple treatments in the treatment arm (combined with copper) - results of individual treatments may vary, trial CTRI/2020/06/026256 (RESCU 002).
Contact: badwera@tmc.gov.in.
Abstract: medRxiv preprint doi: https://doi.org/10.1101/2020.07.21.20151423; this version posted July 29, 2020. The copyright holder for this preprint
(which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
All rights reserved. No reuse allowed without permission.
Resveratrol and Copper for treatment of severe COVID-19:
an observational study (RESCU 002)
Indraneel Mittra 1,2, Rosemarie de Souza 3, Rakesh Bhadade 3, Tushar Madke 3
P.D.Shankpal 3, Mohan Joshi 3, Burhanuddin Qayyumi 1,2, Atanu Bhattacharjee 1,2,
Vikram Gota 1,2, Sudeep Gupta 1,2, Pankaj Chaturvedi 1,2, Rajendra Badwe 1,2
1 Tata Memorial Centre, Mumbai, India, 2 Homi Bhabha National Institute, Mumbai,
India, 3 BYL Nair Charitable Hospital, Mumbai, India
Correspondence to:
Rajendra Badwe
Tata Memorial Centre
Mumbai, India.
badwera@tmc.gov.in
NOTE: This preprint reports new research that has not been certified by peer review and should not be used to guide clinical practice.
1
medRxiv preprint doi: https://doi.org/10.1101/2020.07.21.20151423; this version posted July 29, 2020. The copyright holder for this preprint
(which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
All rights reserved. No reuse allowed without permission.
Abstract
Background
To be universally applicable in treatment of severe COVID-19, novel therapies,
especially those with little toxicity and low cost, are urgently needed. We report here
the use of one such therapeutic combination involving two commonly used
nutraceuticals, namely resveratrol and copper in patients with this disease. This study
was prompted by pre-clinical reports that sepsis-related cytokine storm and fatality in
mice can be prevented by oral administration of small quantities of resveratrol and
copper. Since cytokine storm and sepsis are major causes of death in severe COVID19, we retrospectively analyzed outcomes of patients with this condition who had
received resveratrol and copper.
Methods & Findings
Our analysis comprised of 230 patients with severe COVID-19 requiring inhaled
oxygen who were admitted in a single tertiary care hospital in Mumbai between April
1 and May 13 2020. Thirty of these patients received, in addition to standard care,
resveratrol and copper at doses of 5.6 mg and 560 ng, respectively, orally, once every
6 hours, until discharge or death. These doses were based on our pre-clinical studies,
and were nearly 50 times and 2000 times less, respectively, than those recommended
as health supplements. A multivariable-adjusted analysis was used to model the
outcome of death in these patients and evaluate factors associated with this event. A
binary logistic regression analysis was used, with age, sex, presence of comorbidities
and receipt of resveratrol-copper as covariates. Data were updated as of May 30 2020.
The number of deaths in resveratrol-copper and standard care only groups were 7/30
2
medRxiv preprint doi: https://doi.org/10.1101/2020.07.21.20151423; this version posted July 29, 2020. The copyright holder for this preprint
(which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
All rights reserved. No reuse allowed without permission.
(23.3%, 95% CI 8.1%-38.4%) and 89/200 (44.5%, 95% CI 37.6%-51.3%),
respectively. In multivariable analysis, age >50 years [odds ratio (OR)..
DOI record:
{
"DOI": "10.1101/2020.07.21.20151423",
"URL": "http://dx.doi.org/10.1101/2020.07.21.20151423",
"abstract": "<jats:title>Abstract</jats:title><jats:sec><jats:title>Background</jats:title><jats:p>To be universally applicable in treatment of severe COVID-19, novel therapies, especially those with little toxicity and low cost, are urgently needed. We report here the use of one such therapeutic combination involving two commonly used nutraceuticals, namely resveratrol and copper in patients with this disease. This study was prompted by pre-clinical reports that sepsis-related cytokine storm and fatality in mice can be prevented by oral administration of small quantities of resveratrol and copper. Since cytokine storm and sepsis are major causes of death in severe COVID-19, we retrospectively analyzed outcomes of patients with this condition who had received resveratrol and copper.</jats:p></jats:sec><jats:sec><jats:title>Methods & Findings</jats:title><jats:p>Our analysis comprised of 230 patients with severe COVID-19 requiring inhaled oxygen who were admitted in a single tertiary care hospital in Mumbai between April 1 and May 13 2020. Thirty of these patients received, in addition to standard care, resveratrol and copper at doses of 5.6 mg and 560 ng, respectively, orally, once every 6 hours, until discharge or death. These doses were based on our pre-clinical studies, and were nearly 50 times and 2000 times less, respectively, than those recommended as health supplements. A multivariable-adjusted analysis was used to model the outcome of death in these patients and evaluate factors associated with this event. A binary logistic regression analysis was used, with age, sex, presence of comorbidities and receipt of resveratrol-copper as covariates. Data were updated as of May 30 2020. The number of deaths in resveratrol-copper and standard care only groups were 7/30 (23.3%, 95% CI 8.1%-38.4%) and 89/200 (44.5%, 95% CI 37.6%-51.3%), respectively. In multivariable analysis, age >50 years [odds ratio (OR) 2.558, 95% CI 1.454-4.302, <jats:italic>P</jats:italic>=0.0011] and female sex (OR 1.939, 95% CI 1.079-3.482, <jats:italic>P</jats:italic>=0.0267) were significantly associated, while presence of co-morbidities was not significantly associated (OR 0.713, 95% CI 0.405-1.256, <jats:italic>P</jats:italic>=0.2421) with death. There was a trend towards reduction in death in patients receiving resveratrol-copper (OR 0.413, 95% CI 0.164-1.039, <jats:italic>P</jats:italic>= 0.0604).</jats:p></jats:sec><jats:sec><jats:title>Conclusions</jats:title><jats:p>We provide preliminary results of a novel approach to the treatment of severe COVID-19 using a combination of small amounts of commonly used nutraceuticals, which is non-toxic and inexpensive, and therefore could be widely accessible globally. The nearly two-fold reduction in mortality with resveratrol-copper observed in our study needs to be confirmed in a randomized controlled trial.</jats:p></jats:sec>",
"accepted": {
"date-parts": [
[
2020,
7,
29
]
]
},
"author": [
{
"ORCID": "http://orcid.org/0000-0002-5768-3821",
"affiliation": [],
"authenticated-orcid": false,
"family": "Mittra",
"given": "Indraneel",
"sequence": "first"
},
{
"affiliation": [],
"family": "de Souza",
"given": "Rosemarie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bhadade",
"given": "Rakesh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Madke",
"given": "Tushar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shankpal",
"given": "P.D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Joshi",
"given": "Mohan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Qayyumi",
"given": "Burhanuddin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bhattacharjee",
"given": "Atanu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gota",
"given": "Vikram",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gupta",
"given": "Sudeep",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chaturvedi",
"given": "Pankaj",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Badwe",
"given": "Rajendra",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2020,
7,
29
]
],
"date-time": "2020-07-29T14:15:14Z",
"timestamp": 1596032114000
},
"deposited": {
"date-parts": [
[
2021,
1,
17
]
],
"date-time": "2021-01-17T11:25:26Z",
"timestamp": 1610882726000
},
"group-title": "Infectious Diseases (except HIV/AIDS)",
"indexed": {
"date-parts": [
[
2023,
7,
18
]
],
"date-time": "2023-07-18T12:21:06Z",
"timestamp": 1689682866124
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 12,
"issued": {
"date-parts": [
[
2020,
7,
29
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2020.07.21.20151423",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2020,
7,
29
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2020,
7,
29
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference": [
{
"DOI": "10.3390/genes10060407",
"article-title": "Illegitimate and repeated genomic integration of cell-free chromatin in the aetiology of somatic mosaicism, ageing, chronic diseases and cancer",
"doi-asserted-by": "crossref",
"first-page": "407",
"journal-title": "Genes",
"key": "2021011703250841000_2020.07.21.20151423v1.1",
"volume": "10",
"year": "2019"
},
{
"article-title": "Circulating nucleic acids damage DNA of healthy cells by integrating into their genomes",
"first-page": "91",
"journal-title": "Journal of Biosceince",
"key": "2021011703250841000_2020.07.21.20151423v1.2",
"volume": "40",
"year": "2018"
},
{
"article-title": "Evidence for cell-free nucleic acids as continuously arising endogenous DNA mutagens",
"first-page": "15",
"journal-title": "Mutat. Res. - Fundam. Mol. Mech. Mutagen",
"key": "2021011703250841000_2020.07.21.20151423v1.3",
"volume": "794",
"year": "2016"
},
{
"article-title": "Cell-free chromatin from dying cancer cells integrate into genomes of bystander healthy cells to induce DNA damage and inflammation",
"first-page": "1",
"journal-title": "Cell Death Discov",
"key": "2021011703250841000_2020.07.21.20151423v1.4",
"volume": "3",
"year": "2017"
},
{
"DOI": "10.1016/j.mrfmmm.2018.02.002",
"article-title": "Is inflammation a direct response to dsDNA breaks?",
"doi-asserted-by": "crossref",
"first-page": "48",
"journal-title": "Mutat. Res. - Fundam. Mol. Mech. Mutagen",
"key": "2021011703250841000_2020.07.21.20151423v1.5",
"volume": "808",
"year": "2018"
},
{
"DOI": "10.1007/s12038-019-9849-7",
"article-title": "Cell-free chromatin: A newly described mediator of systemic inflammation",
"doi-asserted-by": "crossref",
"first-page": "32",
"journal-title": "J. Bioscience",
"key": "2021011703250841000_2020.07.21.20151423v1.6",
"volume": "44",
"year": "2019"
},
{
"DOI": "10.1371/journal.pone.0232391",
"doi-asserted-by": "publisher",
"key": "2021011703250841000_2020.07.21.20151423v1.7"
},
{
"DOI": "10.1007/s00011-020-01366-6",
"doi-asserted-by": "crossref",
"key": "2021011703250841000_2020.07.21.20151423v1.8",
"unstructured": "Bellinvia S , Edwards CJ , Schisano M , et al. The unleashing of the immune system in COVID-19 and sepsis: the calm before the storm? Inflamm. Research 2020; 1-7."
},
{
"DOI": "10.1016/j.nut.2015.08.017",
"doi-asserted-by": "publisher",
"key": "2021011703250841000_2020.07.21.20151423v1.9"
},
{
"DOI": "10.1016/S0960-894X(98)00585-X",
"article-title": "Resveratrol as a new type of DNA-cleaving agent. Bioorganic Med",
"doi-asserted-by": "crossref",
"first-page": "3187",
"journal-title": "Chem. Lett",
"key": "2021011703250841000_2020.07.21.20151423v1.10",
"volume": "8",
"year": "1998"
},
{
"DOI": "10.1016/j.jtemb.2016.02.006",
"article-title": "Dietary copper and human health: Current evidence and unresolved issues",
"doi-asserted-by": "crossref",
"first-page": "107",
"journal-title": "Journal of Trace Elements in Medicine and Biology",
"key": "2021011703250841000_2020.07.21.20151423v1.11",
"volume": "35",
"year": "2016"
},
{
"DOI": "10.12688/f1000research.7202.1",
"article-title": "A paradoxical relationship between resveratrol and copper (II) with respect to degradation of DNA and RNA",
"doi-asserted-by": "crossref",
"first-page": "1145",
"journal-title": "F1000 Research",
"key": "2021011703250841000_2020.07.21.20151423v1.12",
"volume": "4",
"year": "2015"
},
{
"DOI": "10.1093/annonc/mdx318",
"article-title": "Prevention of chemotherapy toxicity by agents that neutralize or degrade cell-free chromatin",
"doi-asserted-by": "crossref",
"first-page": "2119",
"journal-title": "Ann. Oncol",
"key": "2021011703250841000_2020.07.21.20151423v1.13",
"volume": "28",
"year": "2017"
},
{
"DOI": "10.1038/s41419-018-1181-x",
"article-title": "Prevention of radiation-induced bystander effects by agents that inactivate cell-free chromatin released from irradiated dying cells",
"doi-asserted-by": "crossref",
"first-page": "1142",
"journal-title": "Cell Death Disease",
"key": "2021011703250841000_2020.07.21.20151423v1.14",
"volume": "9",
"year": "2018"
},
{
"key": "2021011703250841000_2020.07.21.20151423v1.15",
"unstructured": "TRANSMAX™ | Biotivia. https://www.biotivia.com/product/transmax/"
},
{
"key": "2021011703250841000_2020.07.21.20151423v1.16",
"unstructured": "Chelated Copper https://carlsonlabs.com/chelated-copper/"
},
{
"DOI": "10.1096/fj.07-9574LSF",
"doi-asserted-by": "publisher",
"key": "2021011703250841000_2020.07.21.20151423v1.17"
},
{
"DOI": "10.1056/NE-JMoa2007016",
"doi-asserted-by": "publisher",
"key": "2021011703250841000_2020.07.21.20151423v1.18"
},
{
"DOI": "10.1056/NEJMoa2015301",
"doi-asserted-by": "crossref",
"key": "2021011703250841000_2020.07.21.20151423v1.19",
"unstructured": "Goldman JD , Lye DCB , Hui DS , et al. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N Engl J Med 2020."
},
{
"DOI": "10.35500/jghs.2020.2.e17",
"doi-asserted-by": "crossref",
"key": "2021011703250841000_2020.07.21.20151423v1.20",
"unstructured": "Joe W , Kumar A , Rajpal S , Mishra US , Subramanian SV . Equal risk, unequal burden? Gender differentials in COVID-19 mortality in India. J Glob Health Sci 2020;2."
}
],
"reference-count": 20,
"references-count": 20,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2020.07.21.20151423"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "Resveratrol and Copper for treatment of severe COVID-19: an observational study (RESCU 002)",
"type": "posted-content"
}