Regional moderate hyperthermia for mild-to-moderate COVID-19 (TherMoCoV study): a randomized controlled trial
et al., Frontiers in Medicine, doi:10.3389/fmed.2023.1256197, TherMoCoV, NCT04363541, Dec 2023
54th treatment shown to reduce risk in
December 2023, now with p = 0.026 from 4 studies.
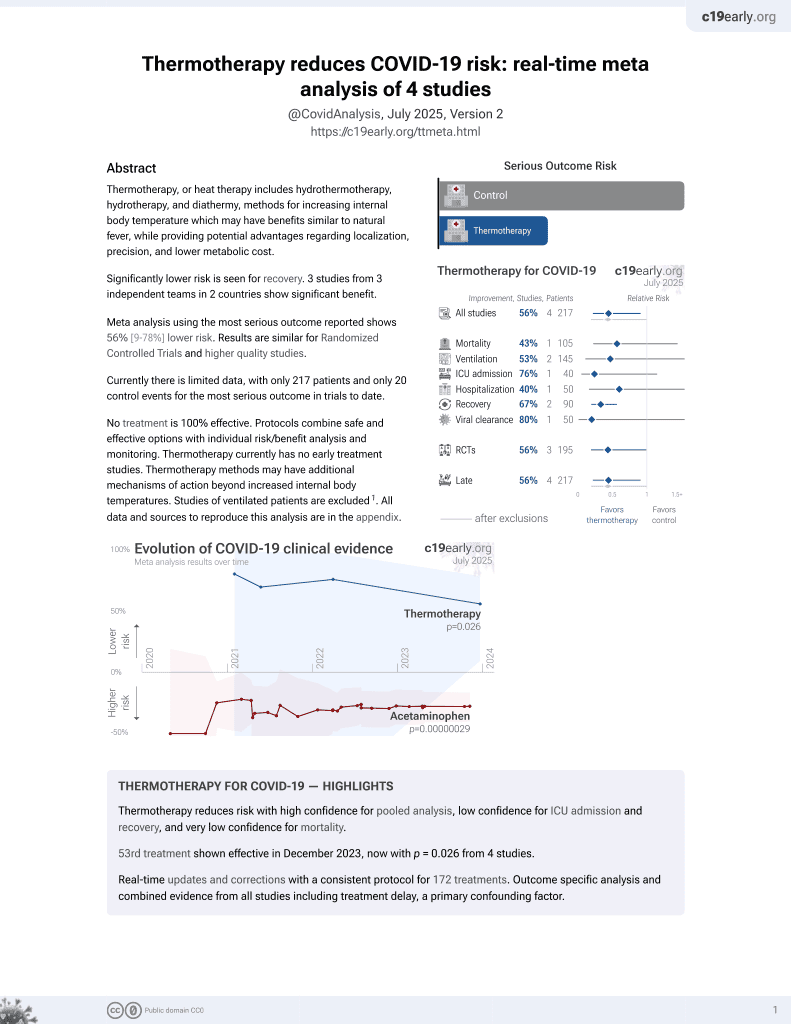
Lower risk for recovery.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 105 hospitalized patients with mild-to-moderate COVID-19, evaluating the efficacy and safety of local thermotherapy (heating pads applied to the chest for 90 minutes twice daily for 5 days) to prevent disease progression, compared to standard care alone. The thermotherapy was well-tolerated with no significant adverse events.
Reduction in NEWS-2 score was significantly faster with treatment. There was lower progression and mortality with treatment, without statistical significance. The study was underpowered due to early termination.
The temperature used may be too low. Lung temperature is expected to be lower than the external skin surface temperature measured on the thorax, due to heat diffusion and dissipation that occurs in transferring thermal energy across the tissue layers of skin, adipose, muscle, connective tissue and bone between the heating pad and the lung.
The treatment group had greater severity at baseline, NEWS-2 7 vs. 5, and PH-COVID-19 high-risk 7.5% vs. 0%.
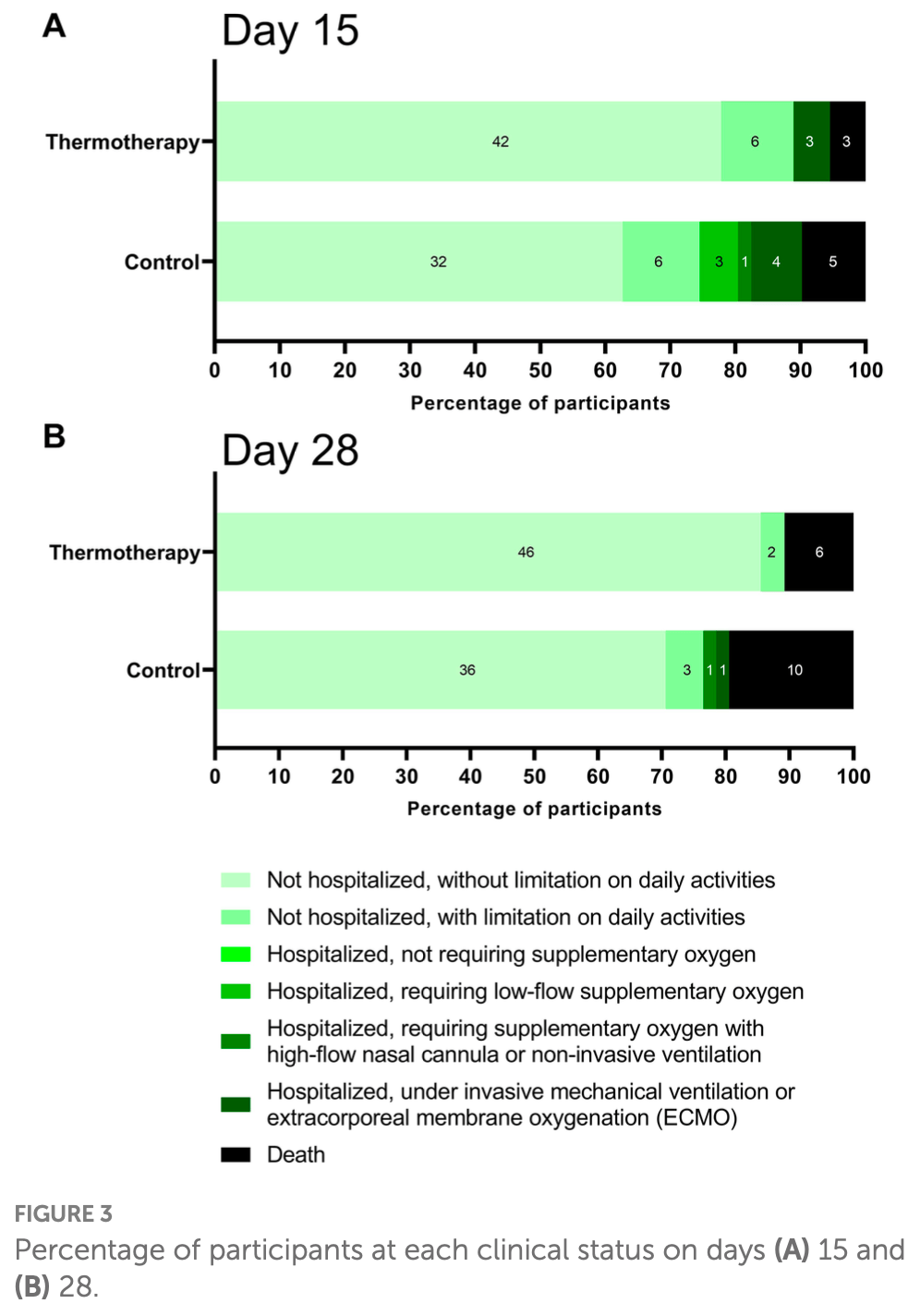
Mortality numbers do not match - Figure 3 shows 10 control deaths at 28 days, while Table 3 shows 8. Percentages reported in Table 3 do not match the counts.
ICU numbers do not match the other data, for example in the control group 6 patients required invasive mechanical ventilation and 10 patients died, but only 3 patients were admitted to the ICU.
|
risk of death, 43.3% lower, RR 0.57, p = 0.28, treatment 6 of 54 (11.1%), control 10 of 51 (19.6%), NNT 12, day 28.
|
|
risk of death, 43.3% lower, RR 0.57, p = 0.48, treatment 3 of 54 (5.6%), control 5 of 51 (9.8%), NNT 24, day 14.
|
|
risk of mechanical ventilation, 21.3% lower, RR 0.79, p = 0.76, treatment 5 of 54 (9.3%), control 6 of 51 (11.8%), NNT 40.
|
|
risk of progression, 17.4% lower, RR 0.83, p = 0.67, treatment 14 of 54 (25.9%), control 16 of 51 (31.4%), NNT 18.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Mancilla-Galindo et al., 22 Dec 2023, Randomized Controlled Trial, Mexico, peer-reviewed, median age 53.0, 15 authors, study period 27 August, 2020 - 23 August, 2021, heating pad, trial NCT04363541 (history) (TherMoCoV).
Contact: ngalindosevilla@hotmail.com.
Regional moderate hyperthermia for mild-to-moderate COVID-19 (TherMoCoV study): a randomized controlled trial
Frontiers in Medicine, doi:10.3389/fmed.2023.1256197
Background: To prevent COVID-19 progression, low-cost alternatives that are available to all patients are needed. Diverse forms of thermotherapy have been proposed to prevent progression to severe/critical COVID-19. Objective: The aim of this study is to evaluate the efficacy and safety of local thermotherapy to prevent disease progression in hospitalized adult patients with mild-to-moderate COVID-19.
Methods: A multicenter, open-label, parallel-group, randomized, adaptive trial is used to evaluate the efficacy and safety of local thermotherapy to prevent disease progression in hospitalized adult patients with mild-tomoderate COVID-19. Eligible hospitalized adult patients with symptoms of COVID-19 with ≤5 days from symptom onset, meeting criteria for mild or moderate COVID-19, were randomly assigned to the intervention consisting of local thermotherapy via an electric heat pad in the thorax (target temperature range 39.5-42°C) continuously for 90 min, twice daily, for 5 days, or standard care. The main outcome was the proportion of patients who progressed to severe-to-critical COVID-19 or death. Patients were randomized in a 1:1 ratio through a centralized computer-generated sequence of minimization with a random component of 20%. Participants and medical staff were not blinded to the intervention.
Ethics statement The studies involving humans were approved by Dirección General de Calidad y Educación en Salud (CEI-DGCES/2020:03.1) and Instituto Nacional de Perinatología (2020-1- 19) . The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
Conflict of interest The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1256197/ full#supplementary-material
References
Asiimwe, Pushpakom, Turner, Kolamunnage-Dona, Jorgensen et al., Cardiovascular drugs and COVID-19 clinical outcomes: a systematic review and meta-analysis of randomized controlled trials, Br J Clin Pharmacol, doi:10.1111/bcp.15331
Basto-Abreu, Carnalla, Torres-Ibarra, Sanchez-Pájaro, Romero-Martínez et al., SARS-CoV-2 seroprevalence and vaccine coverage from august to November 2021: a nationally representative survey in Mexico, J Med Virol, doi:10.1002/jmv.29038
Bautista-Reyes, Werner-Sunderland, Gama, Duran, Medina et al., Health-care policies during the COVID-19 pandemic in Mexico: a continuous case of heterogeneous, reactive, and unequal response, Health Policy Open, doi:10.1016/j.hpopen.2023.100100
Bierer, Meloney, Ahmed, White, Advancing the inclusion of underrepresented women in clinical research, Cell Rep Med, doi:10.1016/j.xcrm.2022.100553
Bonfanti, Mohr, Willms, Bedimo, Gundert et al., Core warming of coronavirus disease 2019 patients undergoing mechanical ventilation: a pilot study, Ther Hypothermia Temp Manag, doi:10.1089/ther.2023.0030
Cohen, Turning up the heat on COVID-19: heat as a therapeutic intervention, F1000Research, doi:10.12688/f1000research.23299.1
Core, R: A language and environment for statistical computing
Dayim, Consort, Create consort diagram
Flahault, Calmy, Costagliola, Drapkina, Eckerle et al., No time for complacency on COVID-19 in Europe, Lancet, doi:10.1016/S0140-6736(23)01012-7
Gandhi, Lynch, Del Rio, Mild or moderate Covid-19, N Engl J Med, doi:10.1056/NEJMcp2009249
Gazel, Yılmaz, Are infectious diseases and microbiology new fields for thermal therapy research?, Int J Hyperth, doi:10.1080/02656736.2018.1440015
Guillaumes, Callaghan, Versión en español del software gratuito OxMaR para minimización y aleatorización de estudios clínicos, Gac Sanit, doi:10.1016/j.gaceta.2018.07.013
Guyatt, Briel, Glasziou, Bassler, Montori, Problems of stopping trials early, BMJ, doi:10.1136/bmj.e3863
Hampton, Eccleston-Turner, Rourke, Switzer, Equity in the pandemic treaty: access and benefit-sharing as a policy device or a rhetorical device?, J Law Med Ethics, doi:10.1017/jme.2023.59
Han, Ma, Li, Liu, Zhao et al., Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors, Emerg Microbes Infect, doi:10.1080/22221751.2020.1770129
Huang, Cheng, Lin, Zhu, Chen, Individualized prediction nomograms for disease progression in mild COVID-19, J Med Virol, doi:10.1002/jmv.25969
Huang, Hernandez, Tang, Dickson, Berenbrok et al., Association between distance to community health care facilities and COVID-19-related mortality across U.S. counties in the COVID-19-vaccine era, BMC Res Notes, doi:10.1186/s13104-023-06366-3
Huang, Li, Shah, Nasb, Ali et al., Efficacy and safety of ultrashort wave diathermy on COVID-19 pneumonia: a pioneering study, Front Med, doi:10.3389/fmed.2023.1149250
Lamontagne, Agarwal, Rochwerg, Siemieniuk, Agoritsas et al., A living WHO guideline on drugs for covid-19, BMJ, doi:10.1136/bmj.m3379
Liikkanen, Laukkanen, Sauna bathing frequency in Finland and the impact of COVID-19, Complement Ther Med, doi:10.1016/j.ctim.2020.102594
Liu, Li, Liu, Liang, Wang et al., Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients, EBioMedicine, doi:10.1016/j.ebiom.2020.102763
Mancilla-Galindo, Galindo-Sevilla, Exploring the rationale for thermotherapy in COVID-19, Int J Hyperth, doi:10.1080/02656736.2021.1883127
Mancilla-Galindo, Vera-Zertuche, Navarro-Cruz, Segura-Badilla, Velázquez et al., Development and validation of the patient history COVID-19 (PH-Covid19) scoring system: a multivariable prediction model of death in Mexican patients with COVID-19, Epidemiol Infect, doi:10.1017/S0950268820002903
Marshall, Murthy, Diaz, Adhikari, Angus et al., A minimal common outcome measure set for COVID-19 clinical research, Lancet Infect Dis, doi:10.1016/S1473-3099(20)30483-7
Masaud, Szasz, Szasz, Ejaz, Anwar et al., A potential bioelectromagnetic method to slow down the progression and prevent the development of ultimate pulmonary fibrosis by COVID-19, Front Immunol, doi:10.3389/fimmu.2020.556335
O'callaghan, OxMaR: open source free software for online minimization and randomization for clinical trials, PLoS ONE, doi:10.1371/journal.pone.0110761
Perez-Padilla, Bouscoulet, Muiño, Marquez, Lopez et al., Prevalance of oxygen desaturation and use of oxygen at home in adults at sea level and at moderate altitude, Eur Respir J, doi:10.1183/09031936.06.00075005
Plata, The black market for covid-19 antiviral drugs, BMJ, doi:10.1136/bmj.o1282
Ramirez, Sanchez, Pirskanen, Hydrothermotherapy in prevention and treatment of mild to moderate cases of COVID-19, Med Hypotheses, doi:10.1016/j.mehy.2020.110363
Ren, Wang, Han, Statins in hospitalized COVID-19 patients: a systematic review and meta-analysis of randomized controlled trials, J Med Virol, doi:10.1002/jmv.28823
Rivera Pérez, Suárez Nadal, Kurschansky, Las, De et al., Saturación de oxígeno en adultos mayores de la Ciudad de México, An Méd
Smith, Latin America roundup: COFEPRIS ends emergency fast tracking for new COVID vaccines, Regulatory Focus
Sánchez-De Prada, Galindo, Fierro, Martínez-García, De Quintana et al., Time evolution of cytokine profiles associated with mortality in COVID-19 hospitalized patients, Front Immunol, doi:10.3389/fimmu.2022.946730
Tian, Wang, Xi, Sun, He et al., Efficacy and safety of short-wave diathermy treatment for moderate COVID-19 patients: a prospective, double-blind, randomized controlled clinical study, Eur J Phys Rehabil Med, doi:10.23736/S1973-9087.21.06892-1
Vargas-Domínguez, Gochicoa-Rangel, Velázquez-Uncal, Mejía-Alfaro, Vázquez-García et al., Pruebas de función respiratoria, ¿cuál y a quién?, Neumol Cir Torax
Vera-Zertuche, Mancilla-Galindo, Tlalpa-Prisco, Aguilar-Alonso, Aguirre-García et al., Obesity is a strong risk factor for short-term mortality and adverse outcomes in Mexican patients with COVID-19: a national observational study, Epidemiol Infect, doi:10.1017/S0950268821001023
Viena, R Foundation for Statistical Computing
Wang, Fan, Horby, Hayden, Li et al., Comparative outcomes of adults hospitalized with seasonal influenza a or B virus infection: application of the 7-category ordinal scale, Open forum. Infect Dis, doi:10.1093/ofid/ofz053
Wu, Mcgoogan, Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China, JAMA, doi:10.1001/jama.2020.2648
Zhang, Wu, Pan, Shen, D-dimer levels and characteristics of lymphocyte subsets, cytokine profiles in peripheral blood of patients with severe COVID-19: a systematic review and meta-analysis, Front Med, doi:10.3389/fmed.2022.988666
DOI record:
{
"DOI": "10.3389/fmed.2023.1256197",
"ISSN": [
"2296-858X"
],
"URL": "http://dx.doi.org/10.3389/fmed.2023.1256197",
"abstract": "<jats:sec><jats:title>Background</jats:title><jats:p>To prevent COVID-19 progression, low-cost alternatives that are available to all patients are needed. Diverse forms of thermotherapy have been proposed to prevent progression to severe/critical COVID-19.</jats:p></jats:sec><jats:sec><jats:title>Objective</jats:title><jats:p>The aim of this study is to evaluate the efficacy and safety of local thermotherapy to prevent disease progression in hospitalized adult patients with mild-to-moderate COVID-19.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>A multicenter, open-label, parallel-group, randomized, adaptive trial is used to evaluate the efficacy and safety of local thermotherapy to prevent disease progression in hospitalized adult patients with mild-to-moderate COVID-19. Eligible hospitalized adult patients with symptoms of COVID-19 with ≤5 days from symptom onset, meeting criteria for mild or moderate COVID-19, were randomly assigned to the intervention consisting of local thermotherapy via an electric heat pad in the thorax (target temperature range 39.5–42°C) continuously for 90 min, twice daily, for 5 days, or standard care. The main outcome was the proportion of patients who progressed to severe-to-critical COVID-19 or death. Patients were randomized in a 1:1 ratio through a centralized computer-generated sequence of minimization with a random component of 20%. Participants and medical staff were not blinded to the intervention.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>One-hundred and five participants (thermotherapy <jats:italic>n</jats:italic> = 54, control <jats:italic>n</jats:italic> = 51) with a median age of 53 (IQR: 41–64) years were included for analysis after the early cessation of recruitment due to the closure of all temporal COVID-19 units (target sample size = 274). The primary outcome of disease progression occurred in 31.4% (16/51) of patients in the control group vs. 25.9% (14/54) of those receiving thermotherapy (risk difference = 5.5%; 95%CI: −11.8–22.7, <jats:italic>p</jats:italic> = 0.54). Thermotherapy was well tolerated with a median total duration of thermotherapy of 900 (IQR: 877.5–900) min. Seven (13.7%) patients in the control group and seven (12.9%) in the thermotherapy group had at least one AE (<jats:italic>p</jats:italic> = 0.9), none of which were causally attributed to the intervention. No statistically significant differences in serum cytokines (IL-1β, IL-6, IL-8, IL-10, IL-17, and IFN-γ) were observed between day 5 and baseline among groups.</jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p>Local thermotherapy was safe and well-tolerated. A non-statistically significant lower proportion of patients who experienced disease progression was found in the thermotherapy group compared to standard care. Local thermotherapy could be further studied as a strategy to prevent disease progression in ambulatory settings.</jats:p><jats:p><jats:bold>Clinical Trial registration</jats:bold>: <jats:ext-link>www.clinicaltrials.gov</jats:ext-link>, identifier: NCT04363541.</jats:p></jats:sec>",
"alternative-id": [
"10.3389/fmed.2023.1256197"
],
"author": [
{
"affiliation": [],
"family": "Mancilla-Galindo",
"given": "Javier",
"sequence": "first"
},
{
"affiliation": [],
"family": "Kammar-García",
"given": "Ashuin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mendoza-Gertrudis",
"given": "María de Lourdes",
"sequence": "additional"
},
{
"affiliation": [],
"family": "García Acosta",
"given": "Javier Michael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nava Serrano",
"given": "Yanira Saralee",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Santiago",
"given": "Oscar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Torres Vásquez",
"given": "Miriam Berenice",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Martínez Martínez",
"given": "Daniela",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fernández-Urrutia",
"given": "Liliana Aline",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Robledo Pascual",
"given": "Julio César",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Narváez Morales",
"given": "Iván Daniel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Velasco-Medina",
"given": "Andrea Aida",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mancilla-Ramírez",
"given": "Javier",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Figueroa-Damián",
"given": "Ricardo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Galindo-Sevilla",
"given": "Norma",
"sequence": "additional"
}
],
"container-title": "Frontiers in Medicine",
"container-title-short": "Front. Med.",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2023,
12,
22
]
],
"date-time": "2023-12-22T04:23:10Z",
"timestamp": 1703218990000
},
"deposited": {
"date-parts": [
[
2023,
12,
22
]
],
"date-time": "2023-12-22T04:23:14Z",
"timestamp": 1703218994000
},
"indexed": {
"date-parts": [
[
2023,
12,
23
]
],
"date-time": "2023-12-23T00:20:19Z",
"timestamp": 1703290819552
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
12,
22
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
12,
22
]
],
"date-time": "2023-12-22T00:00:00Z",
"timestamp": 1703203200000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fmed.2023.1256197/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2023,
12,
22
]
]
},
"published-online": {
"date-parts": [
[
2023,
12,
22
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"key": "ref1",
"year": "2022"
},
{
"key": "ref2",
"year": "2023"
},
{
"DOI": "10.1002/jmv.29038",
"article-title": "SARS-CoV-2 seroprevalence and vaccine coverage from august to November 2021: a nationally representative survey in Mexico",
"author": "Basto-Abreu",
"doi-asserted-by": "publisher",
"first-page": "e29038",
"journal-title": "J Med Virol",
"key": "ref3",
"volume": "95",
"year": "2023"
},
{
"author": "Smith",
"key": "ref4",
"year": "2023"
},
{
"DOI": "10.1017/jme.2023.59",
"article-title": "Equity in the pandemic treaty: access and benefit-sharing as a policy device or a rhetorical device?",
"author": "Hampton",
"doi-asserted-by": "publisher",
"first-page": "217",
"journal-title": "J Law Med Ethics",
"key": "ref5",
"volume": "51",
"year": "2023"
},
{
"DOI": "10.1016/S0140-6736(23)01012-7",
"article-title": "No time for complacency on COVID-19 in Europe",
"author": "Flahault",
"doi-asserted-by": "publisher",
"first-page": "1909",
"journal-title": "Lancet",
"key": "ref6",
"volume": "401",
"year": "2023"
},
{
"DOI": "10.1186/s13104-023-06366-3",
"article-title": "Association between distance to community health care facilities and COVID-19–related mortality across U.S. counties in the COVID-19–vaccine era",
"author": "Huang",
"doi-asserted-by": "publisher",
"first-page": "96",
"journal-title": "BMC Res Notes",
"key": "ref7",
"volume": "16",
"year": "2023"
},
{
"DOI": "10.1136/bmj.m3379",
"article-title": "A living WHO guideline on drugs for covid-19",
"author": "Lamontagne",
"doi-asserted-by": "publisher",
"first-page": "m3379",
"journal-title": "BMJ",
"key": "ref8",
"volume": "370",
"year": "2020"
},
{
"DOI": "10.1136/bmj.o1282",
"article-title": "The black market for covid-19 antiviral drugs",
"author": "Plata",
"doi-asserted-by": "publisher",
"first-page": "o1282",
"journal-title": "BMJ",
"key": "ref9",
"volume": "377",
"year": "2022"
},
{
"DOI": "10.1080/02656736.2021.1883127",
"article-title": "Exploring the rationale for thermotherapy in COVID-19",
"author": "Mancilla-Galindo",
"doi-asserted-by": "publisher",
"first-page": "202",
"journal-title": "Int J Hyperth",
"key": "ref10",
"volume": "38",
"year": "2021"
},
{
"DOI": "10.1080/02656736.2018.1440015",
"article-title": "Are infectious diseases and microbiology new fields for thermal therapy research?",
"author": "Gazel",
"doi-asserted-by": "publisher",
"first-page": "918",
"journal-title": "Int J Hyperth",
"key": "ref11",
"volume": "34",
"year": "2018"
},
{
"DOI": "10.12688/f1000research.23299.1",
"article-title": "Turning up the heat on COVID-19: heat as a therapeutic intervention",
"author": "Cohen",
"doi-asserted-by": "publisher",
"first-page": "292",
"journal-title": "F1000Research",
"key": "ref12",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1016/j.mehy.2020.110363",
"article-title": "Hydrothermotherapy in prevention and treatment of mild to moderate cases of COVID-19",
"author": "Ramirez",
"doi-asserted-by": "publisher",
"first-page": "110363",
"journal-title": "Med Hypotheses",
"key": "ref13",
"volume": "146",
"year": "2021"
},
{
"DOI": "10.1016/j.ctim.2020.102594",
"article-title": "Sauna bathing frequency in Finland and the impact of COVID-19",
"author": "Liikkanen",
"doi-asserted-by": "publisher",
"first-page": "102594",
"journal-title": "Complement Ther Med",
"key": "ref14",
"volume": "2021",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2020.556335",
"article-title": "A potential bioelectromagnetic method to slow down the progression and prevent the development of ultimate pulmonary fibrosis by COVID-19",
"author": "Masaud",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Front Immunol",
"key": "ref15",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1089/ther.2023.0030",
"article-title": "Core warming of coronavirus disease 2019 patients undergoing mechanical ventilation: a pilot study",
"author": "Bonfanti",
"doi-asserted-by": "publisher",
"first-page": "225",
"journal-title": "Ther Hypothermia Temp Manag",
"key": "ref16",
"volume": "13",
"year": "2023"
},
{
"DOI": "10.23736/S1973-9087.21.06892-1",
"article-title": "Efficacy and safety of short-wave diathermy treatment for moderate COVID-19 patients: a prospective, double-blind, randomized controlled clinical study",
"author": "Tian",
"doi-asserted-by": "publisher",
"first-page": "137",
"journal-title": "Eur J Phys Rehabil Med",
"key": "ref17",
"volume": "58",
"year": "2022"
},
{
"DOI": "10.3389/fmed.2023.1149250",
"article-title": "Efficacy and safety of ultra-short wave diathermy on COVID-19 pneumonia: a pioneering study",
"author": "Huang",
"doi-asserted-by": "publisher",
"first-page": "1149250",
"journal-title": "Front Med",
"key": "ref18",
"volume": "10",
"year": "2023"
},
{
"DOI": "10.1001/jama.2020.2648",
"article-title": "Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China",
"author": "Wu",
"doi-asserted-by": "publisher",
"first-page": "1239",
"journal-title": "JAMA",
"key": "ref19",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1056/NEJMcp2009249",
"article-title": "Mild or moderate Covid-19",
"author": "Gandhi",
"doi-asserted-by": "publisher",
"first-page": "1757",
"journal-title": "N Engl J Med",
"key": "ref20",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1002/jmv.25969",
"article-title": "Individualized prediction nomograms for disease progression in mild COVID-19",
"author": "Huang",
"doi-asserted-by": "publisher",
"first-page": "2074",
"journal-title": "J Med Virol",
"key": "ref21",
"volume": "92",
"year": "2020"
},
{
"key": "ref22",
"year": "2020"
},
{
"DOI": "10.1093/ofid/ofz053",
"article-title": "Comparative outcomes of adults hospitalized with seasonal influenza a or B virus infection: application of the 7-category ordinal scale. Open forum",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "ofz053",
"journal-title": "Infect Dis",
"key": "ref23",
"volume": "6",
"year": "2019"
},
{
"key": "ref24",
"volume-title": "National Early Warning Score (NEWS) 2: Standardising the assessment of acute-illness severity in the NHS, vol. 17. Updated report of a working party",
"year": "2017"
},
{
"DOI": "10.1016/S1473-3099(20)30483-7",
"article-title": "A minimal common outcome measure set for COVID-19 clinical research",
"author": "Marshall",
"doi-asserted-by": "publisher",
"first-page": "e192",
"journal-title": "Lancet Infect Dis",
"key": "ref25",
"volume": "20",
"year": "2020"
},
{
"key": "ref26",
"year": "2012"
},
{
"key": "ref27",
"year": "2017"
},
{
"DOI": "10.1136/bmj.e3863",
"article-title": "Problems of stopping trials early",
"author": "Guyatt",
"doi-asserted-by": "publisher",
"first-page": "e3863",
"journal-title": "BMJ",
"key": "ref28",
"volume": "344",
"year": "2012"
},
{
"DOI": "10.1371/journal.pone.0110761",
"article-title": "OxMaR: open source free software for online minimization and randomization for clinical trials",
"author": "O’Callaghan",
"doi-asserted-by": "publisher",
"first-page": "e110761",
"journal-title": "PLoS ONE",
"key": "ref29",
"volume": "9",
"year": "2014"
},
{
"DOI": "10.1016/j.gaceta.2018.07.013",
"article-title": "Versión en español del software gratuito OxMaR para minimización y aleatorización de estudios clínicos",
"author": "Guillaumes",
"doi-asserted-by": "publisher",
"first-page": "395",
"journal-title": "Gac Sanit",
"key": "ref30",
"volume": "33",
"year": "2019"
},
{
"DOI": "10.1017/S0950268820002903",
"article-title": "Development and validation of the patient history COVID-19 (PH-Covid19) scoring system: a multivariable prediction model of death in Mexican patients with COVID-19",
"author": "Mancilla-Galindo",
"doi-asserted-by": "publisher",
"first-page": "e286",
"journal-title": "Epidemiol Infect",
"key": "ref31",
"volume": "148",
"year": "2020"
},
{
"author": "Dayim",
"key": "ref32",
"year": "2023"
},
{
"key": "ref33",
"volume-title": "R: A language and environment for statistical computing [internet]",
"year": "2023"
},
{
"DOI": "10.1017/S0950268821001023",
"article-title": "Obesity is a strong risk factor for short-term mortality and adverse outcomes in Mexican patients with COVID-19: a national observational study",
"author": "Vera-Zertuche",
"doi-asserted-by": "publisher",
"first-page": "e109",
"journal-title": "Epidemiol Infect",
"key": "ref34",
"volume": "149",
"year": "2021"
},
{
"DOI": "10.1002/jmv.28823",
"article-title": "Statins in hospitalized COVID-19 patients: a systematic review and meta-analysis of randomized controlled trials",
"author": "Ren",
"doi-asserted-by": "publisher",
"first-page": "e28823",
"journal-title": "J Med Virol",
"key": "ref35",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.1111/bcp.15331",
"article-title": "Cardiovascular drugs and COVID-19 clinical outcomes: a systematic review and meta-analysis of randomized controlled trials",
"author": "Asiimwe",
"doi-asserted-by": "publisher",
"first-page": "3577",
"journal-title": "Br J Clin Pharmacol",
"key": "ref36",
"volume": "88",
"year": "2022"
},
{
"DOI": "10.1016/j.xcrm.2022.100553",
"article-title": "Advancing the inclusion of underrepresented women in clinical research",
"author": "Bierer",
"doi-asserted-by": "publisher",
"first-page": "100553",
"journal-title": "Cell Rep Med",
"key": "ref37",
"volume": "3",
"year": "2022"
},
{
"DOI": "10.3389/fmed.2022.988666",
"article-title": "D-dimer levels and characteristics of lymphocyte subsets, cytokine profiles in peripheral blood of patients with severe COVID-19: a systematic review and meta-analysis",
"author": "Zhang",
"doi-asserted-by": "publisher",
"first-page": "988666",
"journal-title": "Front Med",
"key": "ref38",
"volume": "9",
"year": "2022"
},
{
"DOI": "10.3389/fimmu.2022.946730",
"article-title": "Time evolution of cytokine profiles associated with mortality in COVID-19 hospitalized patients",
"author": "Sánchez-de Prada",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Front Immunol",
"key": "ref39",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1016/j.ebiom.2020.102763",
"article-title": "Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients",
"author": "Liu",
"doi-asserted-by": "publisher",
"first-page": "102763",
"journal-title": "EBioMedicine",
"key": "ref40",
"volume": "55",
"year": "2020"
},
{
"DOI": "10.1080/22221751.2020.1770129",
"article-title": "Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors",
"author": "Han",
"doi-asserted-by": "publisher",
"first-page": "1123",
"journal-title": "Emerg Microbes Infect",
"key": "ref41",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1016/j.hpopen.2023.100100",
"article-title": "Health-care policies during the COVID-19 pandemic in Mexico: a continuous case of heterogeneous, reactive, and unequal response",
"author": "Bautista-Reyes",
"doi-asserted-by": "publisher",
"first-page": "100100",
"journal-title": "Health Policy Open",
"key": "ref42",
"volume": "5",
"year": "2023"
},
{
"DOI": "10.1183/09031936.06.00075005",
"article-title": "Prevalance of oxygen desaturation and use of oxygen at home in adults at sea level and at moderate altitude",
"author": "Perez-Padilla",
"doi-asserted-by": "publisher",
"first-page": "594",
"journal-title": "Eur Respir J",
"key": "ref43",
"volume": "27",
"year": "2006"
},
{
"article-title": "Pruebas de función respiratoria, ¿cuál y a quién?",
"author": "Vargas-Domínguez",
"first-page": "101",
"journal-title": "Neumol Cir Torax",
"key": "ref44",
"volume": "70",
"year": "2011"
},
{
"article-title": "Saturación de oxígeno en adultos mayores de la Ciudad de México",
"author": "Rivera Pérez",
"first-page": "5",
"journal-title": "An Méd",
"key": "ref45",
"volume": "53",
"year": "2008"
}
],
"reference-count": 45,
"references-count": 45,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.frontiersin.org/articles/10.3389/fmed.2023.1256197/full"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Regional moderate hyperthermia for mild-to-moderate COVID-19 (TherMoCoV study): a randomized controlled trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.3389/crossmark-policy",
"volume": "10"
}