The efficacy and safety of levilimab in severely ill COVID-19 patients not requiring mechanical ventilation: results of a multicenter randomized double-blind placebo-controlled phase III CORONA clinical study
et al., Inflammation Research, doi:10.1007/s00011-021-01507-5, CORONA, NCT04397562, Sep 2021
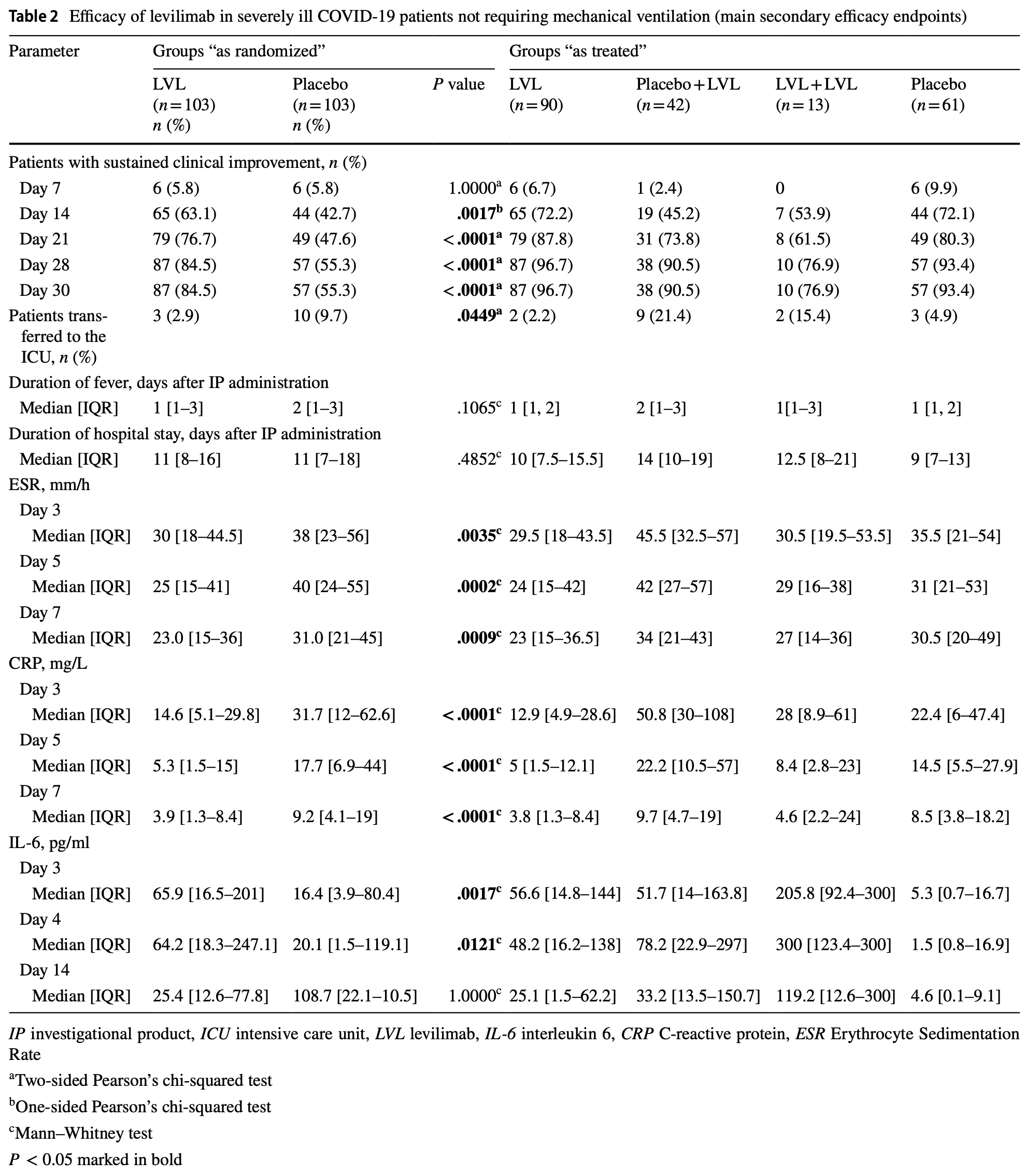
RCT 206 severe COVID-19 patients not requiring mechanical ventilation, showing higher sustained clinical improvement and lower ICU admission with levilimab. There was no difference in mortality.
|
risk of death, no change, RR 1.00, p = 1.00, treatment 4 of 103 (3.9%), control 4 of 103 (3.9%).
|
|
risk of ICU admission, 70.0% lower, RR 0.30, p = 0.08, treatment 3 of 103 (2.9%), control 10 of 103 (9.7%), NNT 15.
|
|
sustained clinical improvement, 65.2% lower, RR 0.35, p < 0.001, treatment 16 of 103 (15.5%), control 46 of 103 (44.7%), NNT 3.4, day 30.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Lomakin et al., 29 Sep 2021, Double Blind Randomized Controlled Trial, placebo-controlled, Russia, peer-reviewed, mean age 58.5, 20 authors, study period April 2020 - August 2020, trial NCT04397562 (history) (CORONA).
Contact: puhtinskaya@biocad.ru.
The efficacy and safety of levilimab in severely ill COVID-19 patients not requiring mechanical ventilation: results of a multicenter randomized double-blind placebo-controlled phase III CORONA clinical study
Inflammation Research, doi:10.1007/s00011-021-01507-5
Objective and design The aim of this double-blind, placebo-controlled, phase III CORONA clinical trial was to evaluate the efficacy and safety of IL-6 receptor inhibitor levilimab (LVL) in subjects with severe COVID-19. Subjects The study included 217 patients. The eligible were men and non-pregnant women aged 18 years or older, hospitalized for severe COVID-19 pneumonia. Treatment 206 subjects were randomized (1:1) to receive single subcutaneous administration of LVL 324 mg or placebo, both in combination with standard of care (SOC). 204 patients received allocated therapy. After the LVL/placebo administration in case of deterioration of symptoms, the investigator could perform a single open-label LVL 324 mg administration as the rescue therapy. Methods The primary efficacy endpoint was the proportion of patients with sustained clinical improvement on the 7-category ordinal scale on Day 14. All efficacy data obtained after rescue therapy administration were considered missing. For primary efficacy analysis, all subjects with missing data were considered non-responders. Results 63.1% and 42.7% of patients in the LVL and in the placebo groups, respectively, achieved sustained clinical improvement on Day 14 (P = .0017). The frequency of adverse drug reactions was comparable between the groups. Conclusion In patients with radiologically confirmed SARS-CoV-2 pneumonia, requiring or not oxygen therapy (but not ventilation) with no signs of other active infection administration of LVL + SOC results in an increase of sustained clinical improvement rate.
Authors and Affiliations Nikita V. Lomakin
References
Cao, Wang, Wen, Liu, Wang et al., A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19, N Engl J Med
Capra, Rossi, Mattioli, Romanelli, Scarpazza et al., Impact of low dose tocilizumab on mortality rate in patients with COVID-19 related pneumonia, Eur J Intern Med
Cevik, Bamford, Ho, COVID-19 pandemic-a focused review for clinicians, Clin Microbiol Infect
Coomes, Haghbayan, Interleukin-6 in Covid-19: a systematic review and meta-analysis, Rev Med Virol
Furlow, COVACTA trial raises questions about tocilizumab's benefit in COVID-19, Lancet Rheumatol
Group, Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial, Lancet
Hermine, Tharaux, Resche-Rigon, Porcher, Ravaud, Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial, JAMA Intern Med
Hojyo, Uchida, Tanaka, Hasebe, Tanaka et al., How COVID-19 induces cytokine storm with high mortality, Inflamm Regen
Investigators, Gordon, Mouncey, Al-Beidh, Rowan et al., Interleukin-6 receptor antagonists in critically Ill patients with Covid-19, N Engl J Med
Khlyabova, Eremeeva, Lutckii, Dokukina, Chernyaeva et al., SAT0040 Safety, tolerability, pharmacokinetics and pharmacodynamics of bcd-089, novel monoclonal anti-il-6 receptor antibody: results from the first-in-human single dose escalation study in healthy volunteers, Ann Rheum Dis
Laing, Lorenc, Molino, Barrio, Das et al., A dynamic COVID-19 immune signature includes associations with poor prognosis, Nat Med
Martinez-Sanz, Ron, Herrera, Perez-Molina, Moreno, Effects of tocilizumab on mortality in hospitalized patients with COVID-19: a multicentre cohort study, Clin Microbiol Infect
Mason, Pathogenesis of COVID-19 from a cell biology perspective, Eur Respir J, doi:10.1183/13993003.00607-2020
Mazurov, Zotkin, Ilivanova, Kropotina, Plaksina et al., FRI0114 efficacy of levilimab, novel monoclonal anti-il-6 receptor antibody, in combination with methotrexate in patients with rheumatoid arthritis: 1 year results of phase 2 AURORA study, Ann Rheum Dis
Mccaw, Tian, Vassy, Ritchie, Lee et al., How to quantify and interpret treatment effects in comparative clinical studies of COVID-19, Ann Intern Med
Rosas, Brau, Waters, Go, Hunter et al., Tocilizumab in hospitalized patients with severe Covid-19 pneumonia, N Engl J Med
Salama, Han, Yau, Reiss, Kramer et al., Tocilizumab in patients hospitalized with Covid-19 pneumonia, N Engl J Med
Somers, Eschenauer, Troost, Golob, Gandhi et al., Tocilizumab for treatment of mechanically ventilated patients with COVID-19, Clin Infect Dis
Stone, Frigault, Serling-Boyd, Fernandes, Harvey et al., Efficacy of tocilizumab in patients hospitalized with Covid-19, N Engl J Med
Wang, Zhang, Du, Du, Zhao, Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial, Lancet
Zeraatkar, Cusano, Martinez, Qasim, Mangala et al., Tocilizumab and sarilumab alone or in combination with corticosteroids for COVID-19: a systematic review and network meta-analysis, doi:10.1101/2021.07.05.21259867
Zhou, Price, Overview on the use of IL-6 agents in the treatment of patients with cytokine release syndrome (CRS) and pneumonitis related to COVID-19 disease, Expert Opin Investig Drugs
DOI record:
{
"DOI": "10.1007/s00011-021-01507-5",
"ISSN": [
"1023-3830",
"1420-908X"
],
"URL": "http://dx.doi.org/10.1007/s00011-021-01507-5",
"abstract": "<jats:title>Abstract</jats:title><jats:sec>\n <jats:title>Objective and design</jats:title>\n <jats:p>The aim of this double-blind, placebo-controlled, phase III CORONA clinical trial was to evaluate the efficacy and safety of IL-6 receptor inhibitor levilimab (LVL) in subjects with severe COVID-19.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Subjects</jats:title>\n <jats:p>The study included 217 patients. The eligible were men and non-pregnant women aged 18 years or older, hospitalized for severe COVID-19 pneumonia.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Treatment</jats:title>\n <jats:p>206 subjects were randomized (1:1) to receive single subcutaneous administration of LVL 324 mg or placebo, both in combination with standard of care (SOC). 204 patients received allocated therapy. After the LVL/placebo administration in case of deterioration of symptoms, the investigator could perform a single open-label LVL 324 mg administration as the rescue therapy.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>The primary efficacy endpoint was the proportion of patients with sustained clinical improvement on the 7-category ordinal scale on Day 14. All efficacy data obtained after rescue therapy administration were considered missing. For primary efficacy analysis, all subjects with missing data were considered non-responders.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>63.1% and 42.7% of patients in the LVL and in the placebo groups, respectively, achieved sustained clinical improvement on Day 14 (<jats:italic>P </jats:italic>= .0017). The frequency of adverse drug reactions was comparable between the groups.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Conclusion</jats:title>\n <jats:p>In patients with radiologically confirmed SARS-CoV-2 pneumonia, requiring or not oxygen therapy (but not ventilation) with no signs of other active infection administration of LVL + SOC results in an increase of sustained clinical improvement rate.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Trail registration</jats:title>\n <jats:p>The trial is registered at the US National Institutes of Health (ClinicalTrials.gov; NCT04397562).</jats:p>\n </jats:sec>",
"alternative-id": [
"1507"
],
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "11 July 2021"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Revised",
"name": "revised",
"order": 2,
"value": "14 September 2021"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 3,
"value": "16 September 2021"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 4,
"value": "29 September 2021"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Conflict of interest",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "The research leading to these results received funding from BIOCAD under Grant Agreement No BCD-089-4/CORONA. Authors Nikita V. Lomakin, Bulat A. Bakirov, Denis N. Protsenko, Vadim I. Mazurov, Gaziyavdibir H. Musaev, Olga M. Moiseeva, Elena S. Pasechnik, Vladimir V. Popov, Elena A. Smolyarchuk, Ivan G. Gordeev, Darya S. Fomina have no conflicts of interest to declare that are relevant to the content of this article. Author Mikhail Yu. Gilyarov received a speaking fee from Boehringer Ingelheim, Bayer, Pfizer и Servier. Authors Anton I. Seleznev, Yulia N. Linkova, Ekaterina A. Dokukina, Polina S. Pukhtinskaia, Anna V. Eremeeva, Maria A. Morozova, Arina V. Zinkina-Orikhan and Anton A. Lutckii, are JSC BIOCAD employees."
},
{
"group": {
"label": "Ethical approval",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "This study was performed in line with the principles of the Declaration of Helsinki of 1964, and its later amendments and was approved by the Central Regulatory Authorities of the Russian Federation (Authorization by the Ministry of Healthcare of the Russian Federation No. 176 of April 22, 2020; Extract from minutes of Ethics Council of Ministry of Health of The Russian Federation No.216, dated 21th of April, 2020) and Ethical Review Boards of each of the participating sites."
},
{
"group": {
"label": "Consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "Informed consent was obtained from all individual participants included in the study."
},
{
"group": {
"label": "Consent to publish",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 5,
"value": "There is no identifiable information in the manuscript. Patients signed informed consent which included information about publishing the analyzed data."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0001-8830-7231",
"affiliation": [],
"authenticated-orcid": false,
"family": "Lomakin",
"given": "Nikita V.",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-3297-1608",
"affiliation": [],
"authenticated-orcid": false,
"family": "Bakirov",
"given": "Bulat A.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5166-3280",
"affiliation": [],
"authenticated-orcid": false,
"family": "Protsenko",
"given": "Denis N.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0797-2051",
"affiliation": [],
"authenticated-orcid": false,
"family": "Mazurov",
"given": "Vadim I.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6550-7975",
"affiliation": [],
"authenticated-orcid": false,
"family": "Musaev",
"given": "Gaziyavdibir H.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7817-3847",
"affiliation": [],
"authenticated-orcid": false,
"family": "Moiseeva",
"given": "Olga M.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6217-2818",
"affiliation": [],
"authenticated-orcid": false,
"family": "Pasechnik",
"given": "Elena S.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1570-2748",
"affiliation": [],
"authenticated-orcid": false,
"family": "Popov",
"given": "Vladimir V.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2615-7167",
"affiliation": [],
"authenticated-orcid": false,
"family": "Smolyarchuk",
"given": "Elena A.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3233-4369",
"affiliation": [],
"authenticated-orcid": false,
"family": "Gordeev",
"given": "Ivan G.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2870-3301",
"affiliation": [],
"authenticated-orcid": false,
"family": "Gilyarov",
"given": "Mikhail Yu",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5083-6637",
"affiliation": [],
"authenticated-orcid": false,
"family": "Fomina",
"given": "Darya S.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9582-7921",
"affiliation": [],
"authenticated-orcid": false,
"family": "Seleznev",
"given": "Anton I.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5463-1022",
"affiliation": [],
"authenticated-orcid": false,
"family": "Linkova",
"given": "Yulia N.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6759-673X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Dokukina",
"given": "Ekaterina A.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5196-6911",
"affiliation": [],
"authenticated-orcid": false,
"family": "Eremeeva",
"given": "Anna V.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9790-8207",
"affiliation": [],
"authenticated-orcid": false,
"family": "Pukhtinskaia",
"given": "Polina S.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7755-7526",
"affiliation": [],
"authenticated-orcid": false,
"family": "Morozova",
"given": "Maria A.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8499-2232",
"affiliation": [],
"authenticated-orcid": false,
"family": "Zinkina-Orikhan",
"given": "Arina V.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2884-1568",
"affiliation": [],
"authenticated-orcid": false,
"family": "Lutckii",
"given": "Anton A.",
"sequence": "additional"
}
],
"clinical-trial-number": [
{
"clinical-trial-number": "nct04397562",
"registry": "10.18810/clinical-trials-gov"
}
],
"container-title": "Inflammation Research",
"container-title-short": "Inflamm. Res.",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2021,
9,
29
]
],
"date-time": "2021-09-29T11:03:30Z",
"timestamp": 1632913410000
},
"deposited": {
"date-parts": [
[
2021,
11,
7
]
],
"date-time": "2021-11-07T14:04:07Z",
"timestamp": 1636293847000
},
"funder": [
{
"award": [
"BCD-089-4/CORONA"
],
"name": "JSC BIOCAD"
}
],
"indexed": {
"date-parts": [
[
2023,
8,
7
]
],
"date-time": "2023-08-07T14:19:01Z",
"timestamp": 1691417941857
},
"is-referenced-by-count": 30,
"issue": "10-12",
"issued": {
"date-parts": [
[
2021,
9,
29
]
]
},
"journal-issue": {
"issue": "10-12",
"published-print": {
"date-parts": [
[
2021,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
9,
29
]
],
"date-time": "2021-09-29T00:00:00Z",
"timestamp": 1632873600000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
9,
29
]
],
"date-time": "2021-09-29T00:00:00Z",
"timestamp": 1632873600000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1007/s00011-021-01507-5.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1007/s00011-021-01507-5/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1007/s00011-021-01507-5.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"page": "1233-1246",
"prefix": "10.1007",
"published": {
"date-parts": [
[
2021,
9,
29
]
]
},
"published-online": {
"date-parts": [
[
2021,
9,
29
]
]
},
"published-print": {
"date-parts": [
[
2021,
12
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1016/j.cmi.2020.04.023",
"author": "M Cevik",
"doi-asserted-by": "publisher",
"first-page": "842",
"issue": "7",
"journal-title": "Clin Microbiol Infect",
"key": "1507_CR1",
"unstructured": "Cevik M, Bamford CGG, Ho A. COVID-19 pandemic-a focused review for clinicians. Clin Microbiol Infect. 2020;26(7):842–7.",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1183/13993003.00607-2020",
"author": "RJ Mason",
"doi-asserted-by": "publisher",
"first-page": "2000607",
"issue": "4",
"journal-title": "Eur Respir J",
"key": "1507_CR2",
"unstructured": "Mason RJ. Pathogenesis of COVID-19 from a cell biology perspective. Eur Respir J. 2020;55(4):2000607. https://doi.org/10.1183/13993003.00607-2020.",
"volume": "55",
"year": "2020"
},
{
"DOI": "10.1186/s41232-020-00146-3",
"author": "S Hojyo",
"doi-asserted-by": "publisher",
"first-page": "37",
"journal-title": "Inflamm Regen",
"key": "1507_CR3",
"unstructured": "Hojyo S, Uchida M, Tanaka K, Hasebe R, Tanaka Y, Murakami M, et al. How COVID-19 induces cytokine storm with high mortality. Inflamm Regen. 2020;40:37.",
"volume": "40",
"year": "2020"
},
{
"DOI": "10.1016/S2665-9913(20)30313-1",
"author": "B Furlow",
"doi-asserted-by": "publisher",
"first-page": "e592",
"issue": "10",
"journal-title": "Lancet Rheumatol.",
"key": "1507_CR4",
"unstructured": "Furlow B. COVACTA trial raises questions about tocilizumab’s benefit in COVID-19. Lancet Rheumatol. 2020;2(10):e592.",
"volume": "2",
"year": "2020"
},
{
"DOI": "10.1038/s41591-020-1038-6",
"author": "AG Laing",
"doi-asserted-by": "publisher",
"first-page": "1623",
"issue": "10",
"journal-title": "Nat Med",
"key": "1507_CR5",
"unstructured": "Laing AG, Lorenc A, Del Molino Del Barrio I, Das A, Fish M, Monin L, et al. A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat Med. 2020;26(10):1623–35.",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1080/13543784.2020.1840549",
"author": "Z Zhou",
"doi-asserted-by": "publisher",
"first-page": "1407",
"issue": "12",
"journal-title": "Expert Opin Investig Drugs",
"key": "1507_CR6",
"unstructured": "Zhou Z, Price CC. Overview on the use of IL-6 agents in the treatment of patients with cytokine release syndrome (CRS) and pneumonitis related to COVID-19 disease. Expert Opin Investig Drugs. 2020;29(12):1407–12.",
"volume": "29",
"year": "2020"
},
{
"author": "P Khlyabova",
"first-page": "884",
"issue": "2",
"journal-title": "Ann Rheum Dis",
"key": "1507_CR7",
"unstructured": "Khlyabova P, Eremeeva A, Lutckii A, Dokukina E, Chernyaeva E, Ivanov R. SAT0040 Safety, tolerability, pharmacokinetics and pharmacodynamics of bcd-089, novel monoclonal anti-il-6 receptor antibody: results from the first-in-human single dose escalation study in healthy volunteers. Ann Rheum Dis. 2018;77(2):884–5.",
"volume": "77",
"year": "2018"
},
{
"author": "V Mazurov",
"first-page": "637",
"issue": "1",
"journal-title": "Ann Rheum Dis",
"key": "1507_CR8",
"unstructured": "Mazurov V, Zotkin E, Ilivanova E, Kropotina T, Plaksina T, Nesmeyanova O, et al. FRI0114 efficacy of levilimab, novel monoclonal anti-il-6 receptor antibody, in combination with methotrexate in patients with rheumatoid arthritis: 1 year results of phase 2 AURORA study. Ann Rheum Dis. 2020;79(1):637–8.",
"volume": "79",
"year": "2020"
},
{
"DOI": "10.1002/rmv.2141",
"author": "EA Coomes",
"doi-asserted-by": "publisher",
"first-page": "1",
"issue": "6",
"journal-title": "Rev Med Virol",
"key": "1507_CR9",
"unstructured": "Coomes EA, Haghbayan H. Interleukin-6 in Covid-19: a systematic review and meta-analysis. Rev Med Virol. 2020;30(6):1–9.",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2001282",
"author": "B Cao",
"doi-asserted-by": "publisher",
"first-page": "1787",
"issue": "19",
"journal-title": "N Engl J Med",
"key": "1507_CR10",
"unstructured": "Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787–99.",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)31022-9",
"author": "Y Wang",
"doi-asserted-by": "publisher",
"first-page": "1569",
"issue": "10236",
"journal-title": "Lancet",
"key": "1507_CR11",
"unstructured": "Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–78.",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.7326/M20-4044",
"author": "ZR McCaw",
"doi-asserted-by": "publisher",
"first-page": "632",
"issue": "8",
"journal-title": "Ann Intern Med",
"key": "1507_CR12",
"unstructured": "McCaw ZR, Tian L, Vassy JL, Ritchie CS, Lee CC, Kim DH, et al. How to quantify and interpret treatment effects in comparative clinical studies of COVID-19. Ann Intern Med. 2020;173(8):632–7.",
"volume": "173",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2030340",
"author": "C Salama",
"doi-asserted-by": "publisher",
"first-page": "20",
"issue": "1",
"journal-title": "N Engl J Med",
"key": "1507_CR13",
"unstructured": "Salama C, Han J, Yau L, Reiss WG, Kramer B, Neidhart JD, et al. Tocilizumab in patients hospitalized with Covid-19 pneumonia. N Engl J Med. 2021;384(1):20–30.",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2028836",
"author": "JH Stone",
"doi-asserted-by": "publisher",
"first-page": "2333",
"issue": "24",
"journal-title": "N Engl J Med",
"key": "1507_CR14",
"unstructured": "Stone JH, Frigault MJ, Serling-Boyd NJ, Fernandes AD, Harvey L, Foulkes AS, et al. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020;383(24):2333–44.",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1016/j.ejim.2020.05.009",
"author": "R Capra",
"doi-asserted-by": "publisher",
"first-page": "31",
"journal-title": "Eur J Intern Med",
"key": "1507_CR15",
"unstructured": "Capra R, De Rossi N, Mattioli F, Romanelli G, Scarpazza C, Sormani MP, et al. Impact of low dose tocilizumab on mortality rate in patients with COVID-19 related pneumonia. Eur J Intern Med. 2020;76:31–5.",
"volume": "76",
"year": "2020"
},
{
"DOI": "10.1001/jamainternmed.2020.6820",
"author": "O Hermine",
"doi-asserted-by": "publisher",
"first-page": "32",
"issue": "1",
"journal-title": "JAMA Intern Med",
"key": "1507_CR16",
"unstructured": "Hermine O, Mariette X, Tharaux PL, Resche-Rigon M, Porcher R, Ravaud P, et al. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181(1):32–40.",
"volume": "181",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2100433",
"author": "R-C Investigators",
"doi-asserted-by": "publisher",
"first-page": "1491",
"issue": "16",
"journal-title": "N Engl J Med",
"key": "1507_CR17",
"unstructured": "Investigators R-C, Gordon AC, Mouncey PR, Al-Beidh F, Rowan KM, Nichol AD, et al. Interleukin-6 receptor antagonists in critically Ill patients with Covid-19. N Engl J Med. 2021;384(16):1491–502.",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2028700",
"author": "IO Rosas",
"doi-asserted-by": "publisher",
"first-page": "1503",
"issue": "16",
"journal-title": "N Engl J Med",
"key": "1507_CR18",
"unstructured": "Rosas IO, Brau N, Waters M, Go RC, Hunter BD, Bhagani S, et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021;384(16):1503–16.",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciaa954",
"author": "EC Somers",
"doi-asserted-by": "publisher",
"first-page": "e445",
"issue": "2",
"journal-title": "Clin Infect Dis",
"key": "1507_CR19",
"unstructured": "Somers EC, Eschenauer GA, Troost JP, Golob JL, Gandhi TN, Wang L, et al. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Clin Infect Dis. 2021;73(2):e445–54.",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.1016/j.cmi.2020.09.021",
"author": "J Martinez-Sanz",
"doi-asserted-by": "publisher",
"first-page": "238",
"issue": "2",
"journal-title": "Clin Microbiol Infect",
"key": "1507_CR20",
"unstructured": "Martinez-Sanz J, Muriel A, Ron R, Herrera S, Perez-Molina JA, Moreno S, et al. Effects of tocilizumab on mortality in hospitalized patients with COVID-19: a multicentre cohort study. Clin Microbiol Infect. 2021;27(2):238–43.",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(21)00676-0",
"author": "Group RC",
"doi-asserted-by": "publisher",
"first-page": "1637",
"issue": "10285",
"journal-title": "Lancet",
"key": "1507_CR21",
"unstructured": "Group RC. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637–45.",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1101/2021.07.05.21259867",
"doi-asserted-by": "publisher",
"key": "1507_CR22",
"unstructured": "Zeraatkar D, Cusano E, Martinez JPD, Qasim A, Mangala SO, Kum E, et al. (2021) Tocilizumab and sarilumab alone or in combination with corticosteroids for COVID-19: a systematic review and network meta-analysis. medRxiv. 2021.07.05.21259867. https://doi.org/10.1101/2021.07.05.21259867"
}
],
"reference-count": 22,
"references-count": 22,
"relation": {},
"resource": {
"primary": {
"URL": "https://link.springer.com/10.1007/s00011-021-01507-5"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology",
"Immunology"
],
"subtitle": [],
"title": "The efficacy and safety of levilimab in severely ill COVID-19 patients not requiring mechanical ventilation: results of a multicenter randomized double-blind placebo-controlled phase III CORONA clinical study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "70"
}