PROVENT: Phase 3 Study of Efficacy and Safety of AZD7442 (Tixagevimab/Cilgavimab) for Pre-exposure Prophylaxis of COVID-19 in Adults
et al., Open Forum Infectious Diseases, doi:10.1093/ofid/ofab466.1646, PROVENT, NCT04625725, Dec 2021
42nd treatment shown to reduce risk in
May 2022, now with p = 0.0066 from 19 studies, recognized in 33 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
PrEP RCT with 3,441 tixagevimab/cilgavimab patients and 1,731 control patients, showing lower risk of symptomatic cases with treatment. Followup data is from1.
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for omicron BA.2.75.2, BA.4.6, BQ.1.12, BA.5, BA.2.75, XBB3,4, XBB.1.54, ХВВ.1.9.14, XBB.1.9.3, XBB.1.5.24, XBB.1.16, XBB.2.9, BQ.1.1.45, CL.1, and CH.1.15.
|
risk of death, 85.7% lower, RR 0.14, p = 0.11, treatment 0 of 3,441 (0.0%), control 2 of 1,731 (0.1%), NNT 866, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
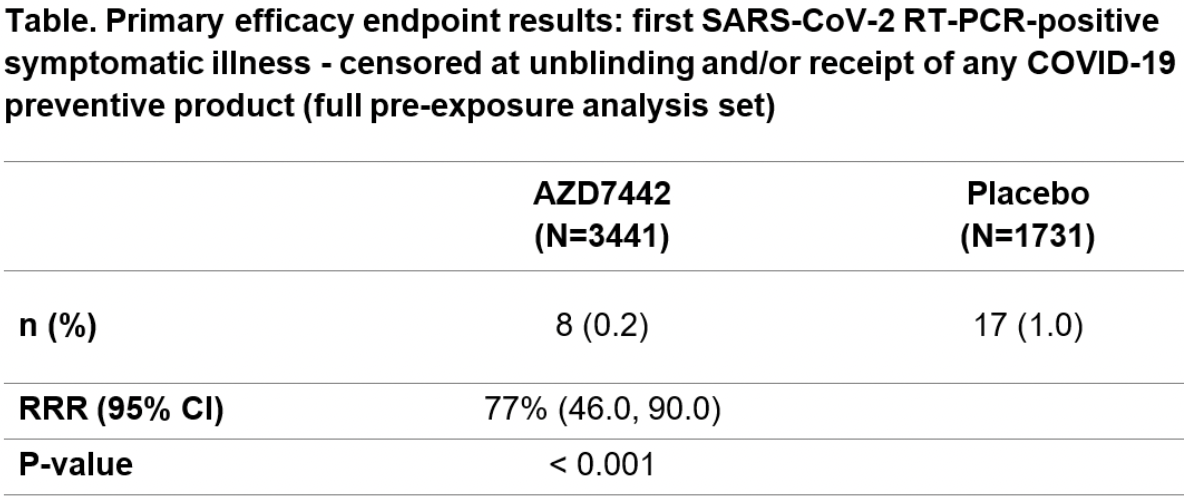
risk of symptomatic case, 76.3% lower, RR 0.24, p < 0.001, treatment 8 of 3,441 (0.2%), control 17 of 1,731 (1.0%), NNT 133.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
2.
Planas et al., Resistance of Omicron subvariants BA.2.75.2, BA.4.6 and BQ.1.1 to neutralizing antibodies, bioRxiv, doi:10.1101/2022.11.17.516888.
3.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
Levin et al., 8 Dec 2021, Double Blind Randomized Controlled Trial, placebo-controlled, multiple countries, preprint, 19 authors, trial NCT04625725 (history) (PROVENT).
Abstract: LB5. PROVENT: Phase 3 Study of Efficacy and Safety of AZD7442 (Tixagevimab/
Cilgavimab) for Pre-exposure Prophylaxis of COVID-19 in Adults
Myron J. Levin, MD1; Andrew Ustianowski, MBBS2;
Stéphane De Wit, MD3; Odile Launay, MD, PhD4; Miles Avila, MPH, GStat5;
Seth Seegobin, PhD5; Alison Templeton, PhD5; Yuan Yuan, PhD6;
Philip Ambery, FRCP5; Rosalinda H. Arends, PhD5; Rohini Beavon, PhD5;
Karen A. Near, MD5; Kelly W. Padilla, PharmD5; Konstantina Psachoulia, PhD5;
Audrey Sharbaugh, PhD5; Katie Streicher, PhD5; Menelas N. Pangalos, PhD5;
Mark T. Esser, PhD5; Robert A. Gasser, Jr., MD5; 1University of Colorado Anschutz
Medical Campus, Aurora, CO; 2North Manchester General Hospital, Manchester,
England, United Kingdom; 3CHU St-Pierre, Brussels, Brussels Hoofdstedelijk Gewest,
Belgium; 4Université de Paris, Inserm F-CRIN I-REIVAC, Paris, Ile-de-France,
France; 5AstraZeneca, Gaithersburg, Maryland; 6AstraZeneca, Gaithersburg, MD,
USA, Gaithersburg, Maryland
Session: 55. Late Breaker Abstracts: COVID-19 Treatment & Prophylaxis
Thursday, September 30, 2021: 6:15 PM
Background. Vaccines effectively prevent COVID-19, but some individuals
have medical comorbidities or receive therapies that impair their immune response
to vaccination, or are ineligible for vaccination. For such individuals who remain at
risk of COVID-19, monoclonal antibodies may provide additional rapid protection.
AZD7442 comprises 2 fully human extended half-life SARS-CoV-2–neutralizing antibodies that bind distinct epitopes of the viral spike protein receptor binding domain.
AZD7442 is in development for the prevention and treatment of COVID-19. Here,
we report primary Phase 3 study results of AZD7442 for pre-exposure prophylaxis of
symptomatic COVID-19.
Methods. PROVENT (NCT04625725) is a Phase 3, 2:1 randomized,
double-blind, placebo-controlled study of a single 300-mg AZD7442 dose (2 intramuscular injections; 150 mg each of tixagevimab and cilgavimab) for symptomatic COVID-19 prevention. Participants were unvaccinated adults (≥ 18 years old)
without prior SARS-CoV-2 infection, who may benefit from immunoprophylaxis with
antibodies due to an increased risk of either inadequate response to vaccination or
SARS-CoV-2 exposure. The primary study endpoints were first case of SARS-CoV-2
RT-PCR-positive symptomatic illness post dose and prior to Day 183 (efficacy), and
safety of AZD7442.
Results. In total, 5197 participants (mean age 53.5 years, 46% female) were randomized and dosed (safety analysis set): AZD7442 n=3460; placebo n=1737. In the
primary efficacy analysis (full pre-exposure analysis set, n=5172), AZD7442 reduced
the risk of developing symptomatic COVID-19 by 77% (95% confidence interval
S810 • OFID 2021:8 (Suppl 1) • Late Breaking Abstracts
46.0, 90.0) vs placebo (P< 0.001) (Table). Adverse events occurred in 35% and 34%
of participants administered AZD7442 and placebo, respectively, and injection site
reactions occurred in 2.4% and 2.1% of participants, respectively (safety analysis set).
There was 1 case of severe/critical COVID-19 and 2 COVID-19–related deaths in
the placebo arm.
Conclusion. The primary study endpoints were met: a one-time dose of AZD7442
demonstrated statistically significant protection against symptomatic COVID-19 and
was well tolerated. AZD7442 is the first long-acting monoclonal antibody combination
that represents a potential new option to augment COVID-19 prevention.
PROVENT funding statement image
Disclosures. Myron J. Levin, MD, GSK..
DOI record:
{
"DOI": "10.1093/ofid/ofab466.1646",
"ISSN": [
"2328-8957"
],
"URL": "http://dx.doi.org/10.1093/ofid/ofab466.1646",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Vaccines effectively prevent COVID-19, but some individuals have medical comorbidities or receive therapies that impair their immune response to vaccination, or are ineligible for vaccination. For such individuals who remain at risk of COVID-19, monoclonal antibodies may provide additional rapid protection. AZD7442 comprises 2 fully human extended half-life SARS-CoV-2–neutralizing antibodies that bind distinct epitopes of the viral spike protein receptor binding domain. AZD7442 is in development for the prevention and treatment of COVID-19. Here, we report primary Phase 3 study results of AZD7442 for pre-exposure prophylaxis of symptomatic COVID-19.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>PROVENT (NCT04625725) is a Phase 3, 2:1 randomized, double-blind, placebo-controlled study of a single 300-mg AZD7442 dose (2 intramuscular injections; 150 mg each of tixagevimab and cilgavimab) for symptomatic COVID-19 prevention. Participants were unvaccinated adults (≥ 18 years old) without prior SARS-CoV-2 infection, who may benefit from immunoprophylaxis with antibodies due to an increased risk of either inadequate response to vaccination or SARS-CoV-2 exposure. The primary study endpoints were first case of SARS-CoV-2 RT-PCR-positive symptomatic illness post dose and prior to Day 183 (efficacy), and safety of AZD7442.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>In total, 5197 participants (mean age 53.5 years, 46% female) were randomized and dosed (safety analysis set): AZD7442 n=3460; placebo n=1737. In the primary efficacy analysis (full pre-exposure analysis set, n=5172), AZD7442 reduced the risk of developing symptomatic COVID-19 by 77% (95% confidence interval 46.0, 90.0) vs placebo (P&lt; 0.001) (Table). Adverse events occurred in 35% and 34% of participants administered AZD7442 and placebo, respectively, and injection site reactions occurred in 2.4% and 2.1% of participants, respectively (safety analysis set). There was 1 case of severe/critical COVID-19 and 2 COVID-19–related deaths in the placebo arm.</jats:p>\n <jats:p />\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusion</jats:title>\n <jats:p>The primary study endpoints were met: a one-time dose of AZD7442 demonstrated statistically significant protection against symptomatic COVID-19 and was well tolerated. AZD7442 is the first long-acting monoclonal antibody combination that represents a potential new option to augment COVID-19 prevention.</jats:p>\n <jats:p>PROVENT funding statement image</jats:p>\n <jats:p />\n </jats:sec>\n <jats:sec>\n <jats:title>Disclosures</jats:title>\n <jats:p>Myron J. Levin, MD, GSK group of companies (Employee, Research Grant or Support) Andrew Ustianowski, MBBS, Vir/GlaxoSmithKline (Advisor or Review Panel member) Stéphane De Wit, MD, Gilead (Grant/Research Support)Janssen (Grant/Research Support)Merck Sharpe & Dohme (Grant/Research Support)ViiV Healthcare (Grant/Research Support) Odile Launay, MD, PhD, AstraZeneca (Grant/Research Support)GlaxoSmithKline (Consultant, Grant/Research Support, Other Financial or Material Support, Data safety monitoring board)Johnson & Johnson (Consultant, Grant/Research Support)Moderna (Consultant)Pfizer (Consultant, Grant/Research Support)Sanofi Pasteur (Consultant, Grant/Research Support) Miles Avila, MPH, GStat, AstraZeneca (Employee, Shareholder) Seth Seegobin, PhD, AstraZeneca (Employee, Shareholder) Alison Templeton, PhD, AstraZeneca (Employee, Shareholder) Yuan Yuan, PhD, AstraZeneca (Employee, Shareholder) Philip Ambery, FRCP, AstraZeneca (Employee, Shareholder) Rosalinda H. Arends, PhD, AstraZeneca (Employee, Shareholder) Rohini Beavon, PhD, AstraZeneca (Employee, Shareholder) Karen A. Near, MD, AstraZeneca (Employee, Shareholder) Kelly W. Padilla, PharmD, AstraZeneca (Employee, Shareholder) Konstantina Psachoulia, PhD, AstraZeneca (Employee, Shareholder) Audrey Sharbaugh, PhD, AstraZeneca (Employee, Shareholder) Katie Streicher, PhD, AstraZeneca (Employee, Shareholder) Menelas N. Pangalos, PhD, AstraZeneca (Employee, Shareholder) Mark T. Esser, PhD, AstraZeneca (Employee, Shareholder) Robert A. Gasser, Jr., MD, AstraZeneca (Employee, Shareholder)</jats:p>\n </jats:sec>",
"author": [
{
"affiliation": [
{
"name": "University of Colorado Anschutz Medical Campus, Aurora, CO"
}
],
"family": "Levin",
"given": "Myron J",
"sequence": "first"
},
{
"affiliation": [
{
"name": "North Manchester General Hospital, Manchester, England, United Kingdom"
}
],
"family": "Ustianowski",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "CHU St-Pierre, Brussels, Brussels Hoofdstedelijk Gewest, Belgium"
}
],
"family": "De Wit",
"given": "Stéphane",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Université de Paris, Inserm F-CRIN I-REIVAC, Paris, Ile-de-France, France"
}
],
"family": "Launay",
"given": "Odile",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "AstraZeneca, Gaithersburg, Maryland"
}
],
"family": "Avila",
"given": "Miles",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "AstraZeneca, Gaithersburg, Maryland"
}
],
"family": "Seegobin",
"given": "Seth",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "AstraZeneca, Gaithersburg, Maryland"
}
],
"family": "Templeton",
"given": "Alison",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "AstraZeneca, Gaithersburg, MD, USA, Gaithersburg, Maryland"
}
],
"family": "Yuan",
"given": "Yuan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "AstraZeneca, Gaithersburg, Maryland"
}
],
"family": "Ambery",
"given": "Philip",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "AstraZeneca, Gaithersburg, Maryland"
}
],
"family": "Arends",
"given": "Rosalinda H",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "AstraZeneca, Gaithersburg, Maryland"
}
],
"family": "Beavon",
"given": "Rohini",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "AstraZeneca, Gaithersburg, Maryland"
}
],
"family": "Near",
"given": "Karen A",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "AstraZeneca, Gaithersburg, Maryland"
}
],
"family": "Padilla",
"given": "Kelly W",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "AstraZeneca, Gaithersburg, Maryland"
}
],
"family": "Psachoulia",
"given": "Konstantina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "AstraZeneca, Gaithersburg, Maryland"
}
],
"family": "Sharbaugh",
"given": "Audrey",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "AstraZeneca, Gaithersburg, Maryland"
}
],
"family": "Streicher",
"given": "Katie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "AstraZeneca, Gaithersburg, Maryland"
}
],
"family": "Pangalos",
"given": "Menelas N",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "AstraZeneca, Gaithersburg, Maryland"
}
],
"family": "Esser",
"given": "Mark T",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "AstraZeneca, Gaithersburg, Maryland"
}
],
"family": "Gasser",
"given": "Robert A",
"sequence": "additional"
}
],
"container-title": [
"Open Forum Infectious Diseases"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
12,
5
]
],
"date-time": "2021-12-05T10:09:10Z",
"timestamp": 1638698950000
},
"deposited": {
"date-parts": [
[
2021,
12,
5
]
],
"date-time": "2021-12-05T10:26:41Z",
"timestamp": 1638700001000
},
"indexed": {
"date-parts": [
[
2022,
2,
15
]
],
"date-time": "2022-02-15T22:28:50Z",
"timestamp": 1644964130780
},
"is-referenced-by-count": 2,
"issn-type": [
{
"type": "electronic",
"value": "2328-8957"
}
],
"issue": "Supplement_1",
"issued": {
"date-parts": [
[
2021,
11,
1
]
]
},
"journal-issue": {
"issue": "Supplement_1",
"published-print": {
"date-parts": [
[
2021,
12,
4
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 33,
"start": {
"date-parts": [
[
2021,
12,
4
]
],
"date-time": "2021-12-04T00:00:00Z",
"timestamp": 1638576000000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/ofid/article-pdf/8/Supplement_1/S810/41521299/ofab466.1646.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/ofid/article-pdf/8/Supplement_1/S810/41521299/ofab466.1646.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"page": "S810-S810",
"prefix": "10.1093",
"published": {
"date-parts": [
[
2021,
11,
1
]
]
},
"published-online": {
"date-parts": [
[
2021,
12,
4
]
]
},
"published-other": {
"date-parts": [
[
2021,
11,
1
]
]
},
"published-print": {
"date-parts": [
[
2021,
12,
4
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference-count": 0,
"references-count": 0,
"relation": {},
"score": 1,
"short-container-title": [],
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Oncology"
],
"subtitle": [],
"title": [
"LB5. PROVENT: Phase 3 Study of Efficacy and Safety of AZD7442 (Tixagevimab/Cilgavimab) for Pre-exposure Prophylaxis of COVID-19 in Adults"
],
"type": "journal-article",
"volume": "8"
}
