Effects of Siltuximab Versus Corticosteroids in Preventing COVID−19 Pneumonia Disease Progression: Multicentre, Open‐Label, Randomized Clinical Trial
et al., Clinical and Translational Science, doi:10.1111/cts.70491, NCT04329650, Feb 2026
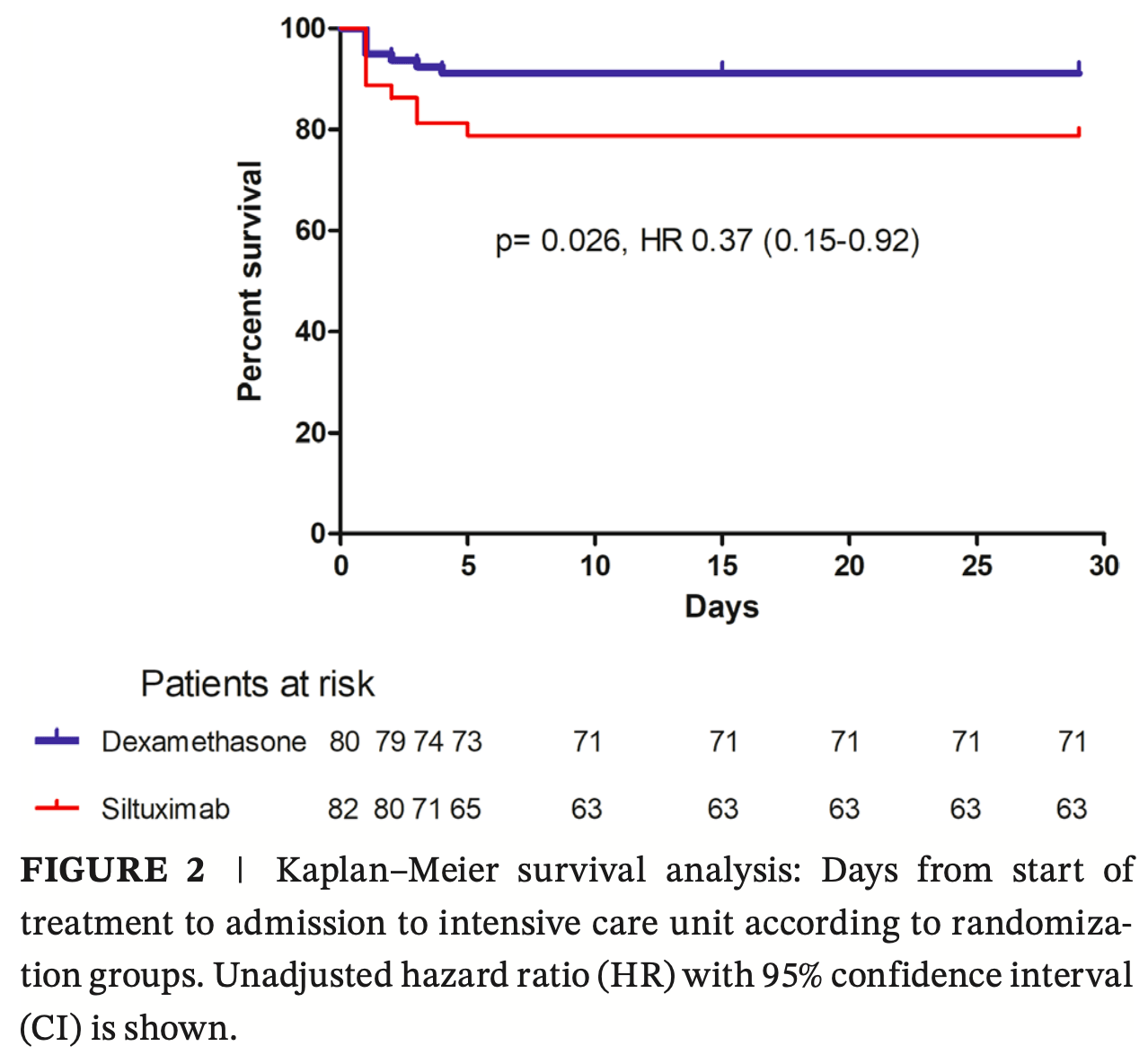
RCT 162 hospitalized COVID-19 pneumonia patients showing harm with siltuximab compared to corticosteroids.
Leal et al., 5 Feb 2026, retrospective, Spain, peer-reviewed, 26 authors, study period 15 April, 2020 - 30 January, 2021, trial NCT04329650 (history).
Contact: lorna.leal@gmail.com.
Effects of Siltuximab Versus Corticosteroids in Preventing COVID −19 Pneumonia Disease Progression: Multicentre, Open‐Label, Randomized Clinical Trial
Clinical and Translational Science, doi:10.1111/cts.70491
In 2020, COVID-19 caused a global health crisis, prompting research efforts and accelerating drug development. As part of this response, we conducted a phase 2b, multicentre, open-label, randomized (1:1) clinical trial to compare the effects of siltuximab versus corticosteroids on disease progression in hospitalized adults with COVID-19 pneumonia. Between April 2020 and January 2021, 82 patients were randomized to receive siltuximab and 80 corticosteroids (20 methylprednisolone and 60 dexamethasone). Median (IQR) age was 61 years (50-72). Nineteen patients allocated to siltuximab were admitted to the intensive care unit compared to eight receiving corticosteroids (p = 0.025). Corticosteroid treatment was independently associated with a higher risk of avoiding intensive care unit admission (2.7; 95% CI, 1.11-6.62), lower risk of requiring mechanical ventilation (0.43; 95% CI, 0.20-0.93) and shorter hospitalization duration (p = 0.008). Mortality was similar between arms (p = 0.675). Twenty-eight patients receiving siltuximab required rescue therapy while only 5 receiving corticosteroids (p < 0.001). Furthermore, patients receiving corticosteroids had a 53% lower risk of confirmed bacterial or fungal invasive infections (RR 0.47; 95% CI, 0.23-0.95; p = 0.0096). Our results show that initial treatment with corticosteroids was more effective than siltuximab in preventing disease progression, reducing intensive care unit admission, mechanical ventilation, with shorter hospital stays, and fewer infection in COVID-19 pneumonia. Despite numerous challenges, these findings provide valuable insights into optimizing therapeutic strategies, supporting corticosteroids as a preferred treatment over siltuximab in this setting.
Author Contributions Lorna Leal, María del Puerto Bernoy González, Sabina Herrera, and Felipe García wrote the manuscript. Lorna Leal, Roger Paredes, Clara Castán, David Dalmau, Alex Soriano, Felipe García, Judit Pich, José Muñóz, Alex Almuedo, Sergio Prieto-González, Pedro Castro, Maria Angeles Marcos, Montse Tuset, and José Antonio Martínez designed the research. Lorna Leal, María del Puerto Bernoy González, Clara Castán, Fernanda Meira, Gerard Dueñas, Judit Pich, Elisa de Lazzari, Marta Hernández-Meneses, Verónica Rico, Nicole García-Pouton, Daiana Agüero, Adrià Tomé, José Muñóz, Alex Almuedo, Sergio Prieto-González, Pedro Castro, Roger Paredes, Cristina Carbonell, and Felipe García performed the research. Felipe García analyzed the data. All authors revised the paper critically for important scientific and intellectual content. All authors approved the final version and made the decision to submit for publication.
Conflicts of Interest Lorna Leal has received consulting fees from HIPRA, honoraria for lectures from Gilead Sciences, educational/training fees from Gilead Sciences and ViiV Healthcare, institutional grants from HIPRA, AstraZeneca B.V. and Janssen Pharmaceuticals Companies of Johnson & Johnson, none related to this work. EdL participates in the Data Safety Monitoring Board for HIPRA. Pedro Castro has received honoraria for scientific collaboration and lecture from Pfizer, MSD, Gilead and AbbVie, and has participated in Advisory Boards with Alexion,..
References
Albuquerque, Eckert, Tramujas, Effect of Tocilizumab, Sarilumab, and Baricitinib on Mortality Among Patients Hospitalized for COVID-19 Treated With Corticosteroids: A Systematic Review and Meta-Analysis, Clinical Microbiology and Infection
Alqahtani, Albilal, Mahmoud, Outcomes Associated With Tocilizumab With or Without Corticosteroid Versus Dexamethasone for Treatment of Patients With Severe to Critical COVID-19 Pneumonia, Journal of Infection and Public Health
Atal, Fatima, IL-6 Inhibitors in the Treatment of Serious COVID-19: A Promising Therapy?, Pharmaceutical Medicine
Bartoletti, Azap, Barac, ESCMID COVID-19 Living Guidelines: Drug Treatment and Clinical Management, Clinical Microbiology and Infection
Bhimraj, Morgan, Shumaker, Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients With Coronavirus Disease 2019 (COVID-19), Clinical Infectious Diseases
Borczuk, Yantiss, The Pathogenesis of Coronavirus-19 Disease, Journal of Biomedical Science
Camou, Issa, Hessamfar, Is Tocilizumab Plus Dexamethasone Associated With Superinfection in Critically Ill COVID-19 Patients?, Journal of Clinical Medicine
Crespillo, Moreno, Antiviral Therapy and Immunotherapy of COVID-19, Revista Española de Quimioterapia
Declercq, Van Damme, Leeuw, Effect of Anti-Interleukin Drugs in Patients With COVID-19 and Signs of Cytokine Release Syndrome (COV-AID): A Factorial, Randomised, Controlled Trial, Lancet Respiratory Medicine
Francisco, Gayo, Zaragozá, A New Approach to the Management of COVID-19. Antagonists of IL-6: Siltuximab, Advances in Therapy
Gritti, Raimondi, Bottazzi, Siltuximab Downregulates Interleukin-8 and Pentraxin 3 to Improve Ventilatory Status and Survival in Severe COVID-19, Leukemia
Hermine, Mariette, Porcher, Tocilizumab Plus Dexamethasone Versus Dexamethasone in Patients With Moderate-To-Severe COVID-19 Pneumonia: A Randomized Clinical Trial From the CORIMUNO-19 Study Group Articles, EClinicalMedicine
Kimmig, Wu, Gold, IL-6 Inhibition in Critically Ill COVID-19 Patients Is Associated With Increased Secondary Infections, Frontiers in Medicine
Kozlakidis, Cappellesso, Abufaraj, Use of Corticosteroids in Coronavirus Disease 2019 Pneumonia: A Systematic Review of the Literature, Pneumonia: A Systematic Review of the Literature Front Med
Lamers, Haagmans, SARS-CoV-2 Pathogenesis, Nature Reviews. Microbiology
Li, He, Qian, Liu, Overview of the Pathogenesis of COVID-19, Experimental and Therapeutic Medicine
Mastrorosa, Gagliardini, Segala, Sarilumab Plus Standard of Care vs Standard of Care for the Treatment of Severe COVID-19: A Phase 3, Randomized, Open-Labeled, Multi-Center Study (ESCAPE Study), EClinicalMedicine
Meira, Albiach, Carbonell, Experience With the Use of Siltuximab in Patients With SARS-CoV-2 Infection, Revista Española de Quimioterapia
Moreno, Solá, Ugarte, Gonzalez-Cordón, Laguno et al., References
Naik, Puri, Kajal, High-Dose Dexamethasone Versus Tocilizumab in Moderate to Severe COVID-19 Pneumonia: A Randomized Controlled Trial, Cureus
Rubin, Longo, Baden, Interleukin-6 Receptor Inhibition in Covid-19 -Cooling the Inflammatory Soup, New England Journal of Medicine
Sahraei, Panahi, Solhjoukhah, The Efficacy of High-Dose Pulse Therapy vs. Low-Dose Intravenous Methylprednisolone on Severe to Critical COVID-19 Clinical Outcomes: A Randomized Clinical Trial, Iranian Journal of Pharmaceutical Research
Shankar-Hari, Vale, Godolphin, Association Between Administration of IL-6 Antagonists and Mortality Among Patients Hospitalized for COVID-19: A Meta-Analysis, JAMA
Siddiqi, Mehra, COVID-19 Illness in Native and Immunosuppressed States: A Clinical-Therapeutic Staging Proposal, Journal of Heart and Lung Transplantation
Sterne, Murthy, Diaz, Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19, JAMA
Stone, Frigault, Serling-Boyd, Efficacy of Tocilizumab in Patient Hospitalized With Covid-19, New England Journal of Medicine
Tetaj, Capone, Stazi, Epidemiology of Ventilator-Associated Pneumonia in ICU COVID-19 Patients: An Alarming High Rate of Multidrug-Resistant Bacteria, Journal of Anesthesia, Analgesia and Critical Care
The, Investigators, Interleukin-6 Receptor Antagonists in Critically Ill Patients With Covid-19, New England Journal of Medicine
Veiga, Prats, Farias, Effect of Tocilizumab on Clinical Outcomes at 15 Days in Patients With Severe or Critical Coronavirus Disease 2019: Randomised Controlled Trial, British Medical Journal
Wang, Jiang, He, A Retrospective Cohort Study of Methylprednisolone Therapy in Severe Patients With COVID-19 Pneumonia, Signal Transduction and Targeted Therapy
DOI record:
{
"DOI": "10.1111/cts.70491",
"ISSN": [
"1752-8054",
"1752-8062"
],
"URL": "http://dx.doi.org/10.1111/cts.70491",
"abstract": "<jats:title>ABSTRACT</jats:title>\n <jats:p>\n In 2020, COVID‐19 caused a global health crisis, prompting research efforts and accelerating drug development. As part of this response, we conducted a phase 2b, multicentre, open‐label, randomized (1:1) clinical trial to compare the effects of siltuximab versus corticosteroids on disease progression in hospitalized adults with COVID‐19 pneumonia. Between April 2020 and January 2021, 82 patients were randomized to receive siltuximab and 80 corticosteroids (20 methylprednisolone and 60 dexamethasone). Median (IQR) age was 61 years (50–72). Nineteen patients allocated to siltuximab were admitted to the intensive care unit compared to eight receiving corticosteroids (\n <jats:italic>p</jats:italic>\n = 0.025). Corticosteroid treatment was independently associated with a higher risk of avoiding intensive care unit admission (2.7; 95% CI, 1.11–6.62), lower risk of requiring mechanical ventilation (0.43; 95% CI, 0.20–0.93) and shorter hospitalization duration (\n <jats:italic>p</jats:italic>\n = 0.008). Mortality was similar between arms (\n <jats:italic>p</jats:italic>\n = 0.675). Twenty‐eight patients receiving siltuximab required rescue therapy while only 5 receiving corticosteroids (\n <jats:italic>p</jats:italic>\n < 0.001). Furthermore, patients receiving corticosteroids had a 53% lower risk of confirmed bacterial or fungal invasive infections (RR 0.47; 95% CI, 0.23–0.95;\n <jats:italic>p</jats:italic>\n = 0.0096). Our results show that initial treatment with corticosteroids was more effective than siltuximab in preventing disease progression, reducing intensive care unit admission, mechanical ventilation, with shorter hospital stays, and fewer infection in COVID‐19 pneumonia. Despite numerous challenges, these findings provide valuable insights into optimizing therapeutic strategies, supporting corticosteroids as a preferred treatment over siltuximab in this setting.\n </jats:p>",
"alternative-id": [
"10.1111/cts.70491"
],
"article-number": "e70491",
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2025-07-01"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "2026-01-03"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 3,
"value": "2026-02-05"
}
],
"author": [
{
"affiliation": [
{
"name": "Infectious Diseases Department Hospital Clínic Barcelona Barcelona Spain"
},
{
"name": "Institut D'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS) Barcelona Spain"
},
{
"name": "Faculty of Medicine Universitat de Barcelona Barcelona Spain"
}
],
"family": "Leal",
"given": "Lorna",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Infectious Diseases Department Hospital Clínic Barcelona Barcelona Spain"
}
],
"family": "del Puerto Bernoy González",
"given": "María",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Diseases Department Hospital Clínic Barcelona Barcelona Spain"
}
],
"family": "Castán",
"given": "Clara",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-5096-4823",
"affiliation": [
{
"name": "Infectious Diseases Department Hospital Clínic Barcelona Barcelona Spain"
}
],
"authenticated-orcid": false,
"family": "Meira",
"given": "Fernanda",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-7857-9927",
"affiliation": [
{
"name": "Infectious Diseases Department Hospital Clínic Barcelona Barcelona Spain"
}
],
"authenticated-orcid": false,
"family": "Dueñas",
"given": "Gerard",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Trials Unit (CTU) Hospital Clínic Barcelona Barcelona Spain"
}
],
"family": "Pich",
"given": "Judit",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-9991-1127",
"affiliation": [
{
"name": "Infectious Diseases Department Hospital Clínic Barcelona Barcelona Spain"
}
],
"authenticated-orcid": false,
"family": "Hernández‐Meneses",
"given": "Marta",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Diseases Department Hospital Clínic Barcelona Barcelona Spain"
}
],
"family": "Rico",
"given": "Verónica",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Diseases Department Hospital Clínic Barcelona Barcelona Spain"
}
],
"family": "García‐Pouton",
"given": "Nicole",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Diseases Department Hospital Clínic Barcelona Barcelona Spain"
}
],
"family": "Agüero",
"given": "Daiana",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-2614-6545",
"affiliation": [
{
"name": "Infectious Diseases Department Hospital Clínic Barcelona Barcelona Spain"
}
],
"authenticated-orcid": false,
"family": "de Lazzari",
"given": "Elisa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine Hospital Clínic Barcelona Barcelona Spain"
}
],
"family": "Tomé",
"given": "Adrià",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Diseases Department Hospital Clínic Barcelona Barcelona Spain"
}
],
"family": "Herrera",
"given": "Sabina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Faculty of Medicine Universitat de Barcelona Barcelona Spain"
},
{
"name": "Institute for Global Health (ISGlobal) Barcelona Spain"
},
{
"name": "Department of International Health Hospital Clinic of Barcelona Barcelona Spain"
}
],
"family": "Muñóz",
"given": "José",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Faculty of Medicine Universitat de Barcelona Barcelona Spain"
},
{
"name": "Institute for Global Health (ISGlobal) Barcelona Spain"
},
{
"name": "Department of International Health Hospital Clinic of Barcelona Barcelona Spain"
}
],
"family": "Almuedo",
"given": "Alex",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institut D'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS) Barcelona Spain"
},
{
"name": "Faculty of Medicine Universitat de Barcelona Barcelona Spain"
},
{
"name": "Department of Internal Medicine Hospital Clínic Barcelona Barcelona Spain"
},
{
"name": "Department of Autoimmune Diseases Hospital Clínic Barcelona Barcelona Spain"
}
],
"family": "Prieto‐González",
"given": "Sergio",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-6118-8970",
"affiliation": [
{
"name": "Institut D'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS) Barcelona Spain"
},
{
"name": "Faculty of Medicine Universitat de Barcelona Barcelona Spain"
},
{
"name": "Medical Intensive Care Unit Hospital Clínic Barcelona Barcelona Spain"
}
],
"authenticated-orcid": false,
"family": "Castro",
"given": "Pedro",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Faculty of Medicine Universitat de Barcelona Barcelona Spain"
},
{
"name": "Institute for Global Health (ISGlobal) Barcelona Spain"
},
{
"name": "Department of Microbiology Hospital Clinic of Barcelona Barcelona Spain"
}
],
"family": "Marcos",
"given": "Maria Angeles",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institut D'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS) Barcelona Spain"
},
{
"name": "Department of Pharmacy, Division of Medicines Hospital Clínic Barcelona Barcelona Spain"
}
],
"family": "Tuset",
"given": "Montse",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases Service Germans Trias i Pujol University Hospital Badalona Spain"
}
],
"family": "Paredes",
"given": "Roger",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine University Hospital of Salamanca Salamanca Spain"
}
],
"family": "Carbonell",
"given": "Cristina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Mutua Terrassa University Hospital Terrassa Spain"
}
],
"family": "Dalmau",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Diseases Department Hospital Clínic Barcelona Barcelona Spain"
},
{
"name": "Institut D'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS) Barcelona Spain"
},
{
"name": "Faculty of Medicine Universitat de Barcelona Barcelona Spain"
}
],
"family": "Martínez",
"given": "José Antonio",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Diseases Department Hospital Clínic Barcelona Barcelona Spain"
},
{
"name": "Institut D'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS) Barcelona Spain"
},
{
"name": "Faculty of Medicine Universitat de Barcelona Barcelona Spain"
}
],
"family": "Soriano",
"given": "Alex",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Diseases Department Hospital Clínic Barcelona Barcelona Spain"
},
{
"name": "Institut D'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS) Barcelona Spain"
},
{
"name": "Faculty of Medicine Universitat de Barcelona Barcelona Spain"
}
],
"family": "García",
"given": "Felipe",
"sequence": "additional"
},
{
"affiliation": [],
"name": "SILCOR study group",
"sequence": "additional"
}
],
"container-title": "Clinical and Translational Science",
"container-title-short": "Clinical Translational Sci",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"ascpt.onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2026,
2,
5
]
],
"date-time": "2026-02-05T12:57:36Z",
"timestamp": 1770296256000
},
"deposited": {
"date-parts": [
[
2026,
2,
5
]
],
"date-time": "2026-02-05T12:57:39Z",
"timestamp": 1770296259000
},
"indexed": {
"date-parts": [
[
2026,
2,
6
]
],
"date-time": "2026-02-06T03:01:40Z",
"timestamp": 1770346900722,
"version": "3.49.0"
},
"is-referenced-by-count": 0,
"issue": "2",
"issued": {
"date-parts": [
[
2026,
2
]
]
},
"journal-issue": {
"issue": "2",
"published-print": {
"date-parts": [
[
2026,
2
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 4,
"start": {
"date-parts": [
[
2026,
2,
5
]
],
"date-time": "2026-02-05T00:00:00Z",
"timestamp": 1770249600000
}
},
{
"URL": "http://doi.wiley.com/10.1002/tdm_license_1.1",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2026,
2,
1
]
],
"date-time": "2026-02-01T00:00:00Z",
"timestamp": 1769904000000
}
}
],
"link": [
{
"URL": "https://ascpt.onlinelibrary.wiley.com/doi/pdf/10.1111/cts.70491",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://ascpt.onlinelibrary.wiley.com/doi/full-xml/10.1111/cts.70491",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://ascpt.onlinelibrary.wiley.com/doi/pdf/10.1111/cts.70491",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"prefix": "10.1111",
"published": {
"date-parts": [
[
2026,
2
]
]
},
"published-online": {
"date-parts": [
[
2026,
2,
5
]
]
},
"published-print": {
"date-parts": [
[
2026,
2
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1038/s41579-022-00713-0",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_2_1"
},
{
"DOI": "10.3892/etm.2021.10444",
"article-title": "Overview of the Pathogenesis of COVID‐19",
"author": "Li C.",
"doi-asserted-by": "crossref",
"journal-title": "Experimental and Therapeutic Medicine",
"key": "e_1_2_10_3_1",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1186/s12929-022-00872-5",
"article-title": "The Pathogenesis of Coronavirus‐19 Disease",
"author": "Borczuk A. C.",
"doi-asserted-by": "crossref",
"journal-title": "Journal of Biomedical Science",
"key": "e_1_2_10_4_1",
"volume": "29",
"year": "2022"
},
{
"DOI": "10.37201/req/s01.17.2021",
"article-title": "Antiviral Therapy and Immunotherapy of COVID‐19",
"author": "Crespillo C.",
"doi-asserted-by": "crossref",
"first-page": "57",
"journal-title": "Revista Española de Quimioterapia",
"key": "e_1_2_10_5_1",
"volume": "34",
"year": "2021"
},
{
"DOI": "10.1016/j.healun.2020.03.012",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_6_1"
},
{
"DOI": "10.1038/s41392-020-0158-2",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_7_1"
},
{
"article-title": "Use of Corticosteroids in Coronavirus Disease 2019 Pneumonia: A Systematic Review of the Literature",
"author": "Kozlakidis Z.",
"journal-title": "Pneumonia: A Systematic Review of the Literature Front Med",
"key": "e_1_2_10_8_1",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2021436",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_9_1"
},
{
"DOI": "10.1001/jama.2020.17023",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_10_1"
},
{
"key": "e_1_2_10_11_1",
"unstructured": "COVID‐19 Treatment Guidelines https://www.covid19treatmentguidelines.nih.gov/."
},
{
"article-title": "A New Approach to the Management of COVID‐19. Antagonists of IL‐6: Siltuximab",
"author": "Francisco L. V.",
"first-page": "1126",
"journal-title": "Advances in Therapy",
"key": "e_1_2_10_12_1",
"volume": "39",
"year": "2042"
},
{
"DOI": "10.1056/NEJMoa2028836",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_13_1"
},
{
"DOI": "10.1056/NEJMe2103108",
"article-title": "Interleukin‐6 Receptor Inhibition in Covid‐19 — Cooling the Inflammatory Soup",
"author": "Rubin E. J.",
"doi-asserted-by": "crossref",
"first-page": "1564",
"journal-title": "New England Journal of Medicine",
"key": "e_1_2_10_14_1",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1007/s40290-020-00342-z",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_15_1"
},
{
"DOI": "10.37201/req/045.2021",
"article-title": "Experience With the Use of Siltuximab in Patients With SARS‐CoV‐2 Infection",
"author": "Meira F.",
"doi-asserted-by": "crossref",
"first-page": "337",
"journal-title": "Revista Española de Quimioterapia",
"key": "e_1_2_10_16_1",
"volume": "34",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.11330",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_17_1"
},
{
"DOI": "10.1038/s41375-021-01299-x",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_18_1"
},
{
"DOI": "10.1016/S2213-2600(21)00377-5",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_19_1"
},
{
"DOI": "10.1016/j.cmi.2022.07.008",
"article-title": "Effect of Tocilizumab, Sarilumab, and Baricitinib on Mortality Among Patients Hospitalized for COVID‐19 Treated With Corticosteroids: A Systematic Review and Meta‐Analysis",
"author": "Albuquerque A. M.",
"doi-asserted-by": "crossref",
"first-page": "13",
"journal-title": "Clinical Microbiology and Infection",
"key": "e_1_2_10_20_1",
"volume": "29",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2100433",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_21_1"
},
{
"DOI": "10.1016/S0140-6736(21)00676-0",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_22_1"
},
{
"DOI": "10.1136/bmj.n84",
"article-title": "Effect of Tocilizumab on Clinical Outcomes at 15 Days in Patients With Severe or Critical Coronavirus Disease 2019: Randomised Controlled Trial",
"author": "Veiga V. C.",
"doi-asserted-by": "crossref",
"journal-title": "British Medical Journal",
"key": "e_1_2_10_23_1",
"volume": "372",
"year": "2021"
},
{
"DOI": "10.1016/j.eclinm.2023.101895",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_24_1"
},
{
"DOI": "10.1186/s44158-022-00065-4",
"article-title": "Epidemiology of Ventilator‐Associated Pneumonia in ICU COVID‐19 Patients: An Alarming High Rate of Multidrug‐Resistant Bacteria",
"author": "Tetaj N.",
"doi-asserted-by": "crossref",
"journal-title": "Journal of Anesthesia, Analgesia and Critical Care",
"key": "e_1_2_10_25_1",
"volume": "2",
"year": "2022"
},
{
"DOI": "10.3390/jcm11195559",
"article-title": "Is Tocilizumab Plus Dexamethasone Associated With Superinfection in Critically Ill COVID‐19 Patients?",
"author": "Camou F.",
"doi-asserted-by": "crossref",
"first-page": "5559",
"journal-title": "Journal of Clinical Medicine",
"key": "e_1_2_10_26_1",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.3389/fmed.2020.583897",
"article-title": "IL‐6 Inhibition in Critically Ill COVID‐19 Patients Is Associated With Increased Secondary Infections",
"author": "Kimmig L. M.",
"doi-asserted-by": "crossref",
"journal-title": "Frontiers in Medicine",
"key": "e_1_2_10_27_1",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1016/j.eclinm.2022.101362",
"article-title": "Tocilizumab Plus Dexamethasone Versus Dexamethasone in Patients With Moderate‐To‐Severe COVID‐19 Pneumonia: A Randomized Clinical Trial From the CORIMUNO‐19 Study Group Articles",
"author": "Hermine O.",
"doi-asserted-by": "crossref",
"journal-title": "EClinicalMedicine",
"key": "e_1_2_10_28_1",
"volume": "46",
"year": "2022"
},
{
"DOI": "10.1016/j.jiph.2021.11.017",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_29_1"
},
{
"article-title": "High‐Dose Dexamethasone Versus Tocilizumab in Moderate to Severe COVID‐19 Pneumonia: A Randomized Controlled Trial",
"author": "Naik N. B.",
"journal-title": "Cureus",
"key": "e_1_2_10_30_1",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.5812/ijpr-137838",
"article-title": "The Efficacy of High‐Dose Pulse Therapy vs. Low‐Dose Intravenous Methylprednisolone on Severe to Critical COVID‐19 Clinical Outcomes: A Randomized Clinical Trial",
"author": "Sahraei Z.",
"doi-asserted-by": "crossref",
"journal-title": "Iranian Journal of Pharmaceutical Research",
"key": "e_1_2_10_31_1",
"volume": "22",
"year": "2023"
},
{
"DOI": "10.1016/j.cmi.2021.11.007",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_32_1"
},
{
"article-title": "Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients With Coronavirus Disease 2019 (COVID‐19)",
"author": "Bhimraj A.",
"first-page": "e250",
"journal-title": "Clinical Infectious Diseases",
"key": "e_1_2_10_33_1",
"volume": "78",
"year": "2024"
}
],
"reference-count": 32,
"references-count": 32,
"relation": {},
"resource": {
"primary": {
"URL": "https://ascpt.onlinelibrary.wiley.com/doi/10.1111/cts.70491"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Effects of Siltuximab Versus Corticosteroids in Preventing\n <scp>COVID</scp>\n −19 Pneumonia Disease Progression: Multicentre, Open‐Label, Randomized Clinical Trial",
"type": "journal-article",
"update-policy": "https://doi.org/10.1002/crossmark_policy",
"volume": "19"
}