To clarify the safety profile of paracetamol for home-care patients with COVID-19: a real-world cohort study, with nested case–control analysis, in primary care
et al., Internal and Emergency Medicine, doi:10.1007/s11739-022-03054-1, Jul 2022
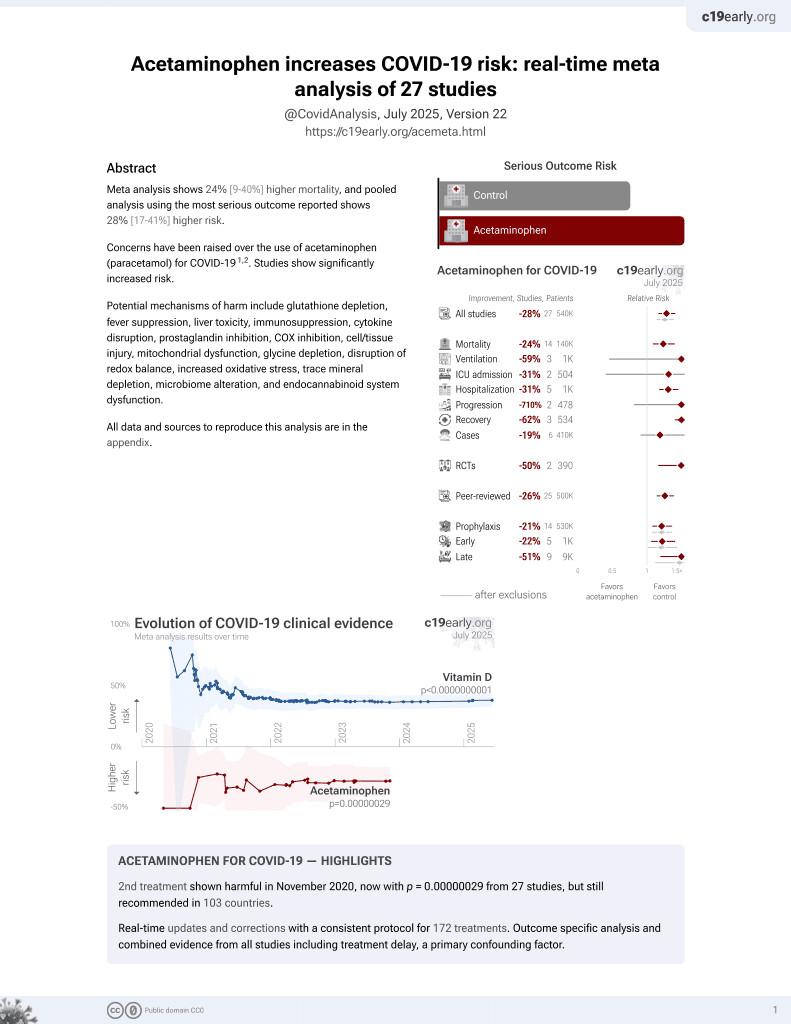
2nd treatment shown to increase risk in
November 2020, now with p = 0.00000029 from 27 studies, but still recommended in 103 countries.
6,400+ studies for
210+ treatments. c19early.org
|
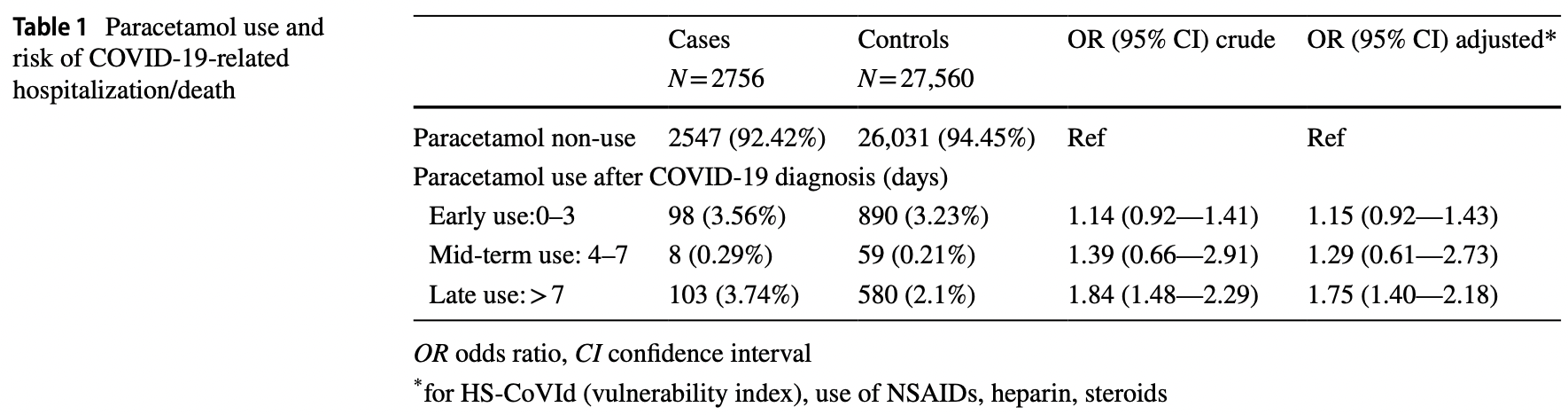
Retrospective paracetamol use with a primary care database in Italy, showing no significant difference in hospitalization/death for use 0-3 and 4-7 days from diagnosis, and significantly higher risk for use >7 days from diagnosis. Confounding by indication may have a greater effect on late usage.
Paracetamol is also known as acetaminophen, Tylenol, Panadol, Calpol, Tempra, Calprofen, Doliprane, Efferalgan, Grippostad C, Dolo, Acamol, Fevadol, Crocin, and Perfalgan.
|
risk of death/hospitalization, 15.0% higher, OR 1.15, p = 0.22, adjusted per study, early use, RR approximated with OR.
|
|
risk of death/hospitalization, 29.0% higher, OR 1.29, p = 0.52, adjusted per study, mid-term use, RR approximated with OR.
|
|
risk of death/hospitalization, 75.0% higher, OR 1.75, p < 0.001, adjusted per study, late use, RR approximated with OR, late treatment result, excluded in exclusion analyses:
substantial unadjusted confounding by indication likely.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Lapi et al., 30 Jul 2022, retrospective, Italy, peer-reviewed, 8 authors.
Contact: lapi.francesco@simg.it.
To clarify the safety profile of paracetamol for home-care patients with COVID-19: a real-world cohort study, with nested case–control analysis, in primary care
Internal and Emergency Medicine, doi:10.1007/s11739-022-03054-1
Background and objective This study aimed to compare the prescribing patterns of paracetamol in COVID-19 with those for similar respiratory conditions and investigated the association between paracetamol use and COVID-19-related hospitalization/death. Methods Using a primary care data source, we conducted a cohort study to calculate the incidence rate of paracetamol use in COVID-19 and for similar respiratory conditions in 2020 and 2019 (i.e. pre-pandemic phase), respectively. In the study cohort, we nested a case-control analyses to investigate the association between paracetamol use and COVID-19-related hospitalizations/deaths. Results Overall, 1554 (33.4 per 1000) and 2566 patients (78.3 per 1000) were newly prescribed with paracetamol to treat COVID-19 or other respiratory conditions, respectively. Those aged 35-44 showed the highest prevalence rate (44.7 or 99.0 per 1000), while the oldest category reported the lowest value (17.8 or 39.8 per 1000). There was no association for early (OR = 1.15; 95% CI: 0.92-1.43) or mid-term (OR = 1.29; 95% CI: 0.61-2.73) users of paracetamol vs. non-users. Instead, the late users of paracetamol showed a statistically significant increased risk of hospitalization/death (OR = 1.75; 95% CI: 1.4-2.2). Conclusions Our findings provide reassuring evidence on the use and safety profile of paracetamol to treat early symptoms of COVID-19 as in other respiratory infections.
Supplementary Information The online version contains supplementary material available at https:// doi. org/ 10. 1007/ s11739-022-03054-1. Authors contribution FL, IG, AR, DF, EM, AM, PLA and CC: have made substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data. FL, EM, IG, DF, PLR and CC: have been involved in drafting the manuscript or revising it critically for important intellectual content. CC: is responsible for the integrity of the work, and he given final approval of the version to be published. Each author should have participated sufficiently in the work to take public responsibility for appropriate portions of the content. FL: agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of interest FL and EM provided consultancies in protocol preparation for epidemiological studies and data analyses for Angelini, Pfizer, and GSK. AR, IG, AM, PLA, and CC provided clinical consultancies for Angelini, Pfizer, and GSK. DF has no conflict of interest to disclose.
Ethical approval According to a by-law on the classification and implementation of observational drug-related research, as issued by the Italian National Drug Agency (an entity belonging to the Italian Ministry of Health), the present study does not require approval by an Ethics Committee in Italy (Italian Drug Agency..
References
Bianchini, Brignoli, Cricelli C VIII Report Health Search
Breslow, Statistics in epidemiology: the case-control study, J Am Stat Assoc, doi:10.1080/01621459.1996.10476660
Collie, Champion, Moultrie, Effectiveness of BNT162b2 vaccine against omicron variant in South Africa, N Engl J Med, doi:10.1056/NEJMC2119270/SUPPL_FILE/NEJMC2119270_DISCLOSURES.PDF
Collins, Reitsma, Altman, Moons, Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement, J Clin Epidemiol, doi:10.1016/j.jclinepi.2014.11.010
Cricelli, Mazzaglia, Samani, Prevalence estimates for chronic diseases in Italy: exploring the differences between self-report and primary care databases, J Pub Health Med
Dimova, Hoet, Dinsdale, Nemery, Acetaminophen decreases intracellular glutathione levels and modulates cytokine production in human alveolar macrophages and type II pneumocytes in vitro, Int J Biochem Cell Biol, doi:10.1016/J.BIOCEL.2005.03.005
Dinnes, Deeks, Berhane, Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection, Cochrane Database Syst Rev, doi:10.1002/14651858.CD013705.PUB2
Dri, How to treat COVID-19 patients at home in the Italian context: an expert opinion, Infect Dis Rep, doi:10.3390/IDR13010028
Elliott, Whitaker, Bodinier, Predictive symptoms for COVID-19 in the community: REACT-1 study of over 1 million people, PLOS Med, doi:10.1371/JOURNAL.PMED.1003777
Enners, Gradl, Kieble, Utilization of drugs with reports on potential efficacy or harm on COVID-19 before, during, and after the first pandemic wave, Pharmacoepidemiol Drug Saf, doi:10.1002/pds.5324
Faillie, Indication bias or protopathic bias?, Br J Clin Pharmacol, doi:10.1111/BCP.12705
Guglielmi, Bellia, Pecchioli, What is the actual epidemiology of familial hypercholesterolemia in Italy? Int J Cardiol, Evidence from a National Primary Care Database, doi:10.1016/j.ijcard.2016.08.269
Hempenius, Groenwold, De Boer, Drug exposure misclassification in pharmacoepidemiology: sources and relative impact, Pharmacoepidemiol Drug Saf, doi:10.1002/pds.5346
Kennon-Mcgill, Mcgill, Extrahepatic toxicity of acetaminophen: critical evaluation of the evidence and proposed mechanisms, J Clin Transl Res
Lapi, Domnich, Marconi, Predicting the risk of severe COVID-19 outcomes in primary care: development and validation of a vulnerability index for equitable allocation of effective vaccines, Expert Rev Vaccines, doi:10.1080/14760584.2022.2019582
Lapi, Simonetti, Michieli, Assessing 5-year incidence rates and determinants of osteoporotic fractures in primary care, Bone, doi:10.1016/j.bone.2011.09.048
Lawrenson, Williams, Farmer, Clinical information for research; the use of general practice databases, J Public Health Med
Mitchell, Jollows, Metabolic activation of drugs to toxic substances, Gastroenterology, doi:10.1016/S0016-5085(75)80025-4
Orsini, Bellocco, Bottai, A tool for deterministic and probabilistic sensitivity analysis of epidemiologic studies, Stata J
Pandolfi, Chirumbolo, Home therapy of COVID-19 at the earliest may greatly prevent hospitalization, Basic Clin Pharmacol Toxicol, doi:10.1111/bcpt.13650
Pandolfi, Chirumbolo, Ricevuti, Home pharmacological therapy in early COVID-19 to prevent hospitalization and reduce mortality: time for a suitable proposal, Basic Clin Pharmacol Toxicol, doi:10.1111/bcpt.13690
Pandolfi, Simonetti, Ricevuti, Chirumbolo, Paracetamol in the home treatment of early COVID-19 symptoms: a possible foe rather than a friend for elderly patients?, J Med Virol
Prada, Santos, Baião, Risk of SARS-CoV-2 Infection and COVID-19 Severity Associated With Exposure to Nonsteroidal Anti-Inflammatory Drugs: Systematic Review and Meta-Analysis, J. Clin, Pharmacol
Ranstam, Multiple P-values and Bonferroni correction, Osteoarthr Cartil, doi:10.1016/j.joca.2016.01.008
Schneeweiss, Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics, Pharmacoepidemiol Drug Saf, doi:10.1002/pds.1200
Suter, Consolaro, Pedroni, A simple, home-therapy algorithm to prevent hospitalisation for COVID-19 patients: a retrospective observational matched-cohort study, doi:10.1016/J.ECLINM.2021.100941
Suter, Consolaro, Pedroni, A simple, hometherapy algorithm to prevent hospitalisation for COVID-19 patients: a retrospective observational matched-cohort study, EClinicalMedicine
Tamim, Monfared, Lelorier, Application of lag-time into exposure definitions to control for protopathic bias, Pharmacoepidemiol Drug Saf, doi:10.1002/PDS.1360
Tuccori, Convertino, Ferraro, The impact of the COVID-19 "Infodemic" on drug-utilization behaviors: implications for pharmacovigilance, Drug Saf, doi:10.1007/S40264-020-00965-W
Vaja, Chan, Ferreira, The COVID-19 ibuprofen controversy: a systematic review of NSAIDs in adult acute lower respiratory tract infections, Br J Clin Pharmacol, doi:10.1111/BCP.14514
DOI record:
{
"DOI": "10.1007/s11739-022-03054-1",
"ISSN": [
"1828-0447",
"1970-9366"
],
"URL": "http://dx.doi.org/10.1007/s11739-022-03054-1",
"alternative-id": [
"3054"
],
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "3 June 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "11 July 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "30 July 2022"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Conflict of interest",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "FL and EM provided consultancies in protocol preparation for epidemiological studies and data analyses for Angelini, Pfizer, and GSK. AR, IG, AM, PLA, and CC provided clinical consultancies for Angelini, Pfizer, and GSK. DF has no conflict of interest to disclose."
},
{
"group": {
"label": "Ethical approval",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "According to a by-law on the classification and implementation of observational drug-related research, as issued by the Italian National Drug Agency (an entity belonging to the Italian Ministry of Health), the present study does not require approval by an Ethics Committee in Italy (Italian Drug Agency note of 3 August 2007). This study followed the principles of the Declaration of Helsinki and compliant with the ENCePP (European Network of Centres for Pharmacoepidemiology and Pharmacovigilance) Guide on Methodological Standards in Pharmacoepidemiology."
},
{
"group": {
"label": "Consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "The database is fully anonymized."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 5,
"value": "Not applicable."
},
{
"label": "Free to read",
"name": "free",
"value": "This content has been made available to all."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-4342-9128",
"affiliation": [],
"authenticated-orcid": false,
"family": "Lapi",
"given": "Francesco",
"sequence": "first"
},
{
"affiliation": [],
"family": "Marconi",
"given": "Ettore",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Grattagliano",
"given": "Ignazio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rossi",
"given": "Alessandro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fornasari",
"given": "Diego",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Magni",
"given": "Alberto",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lora Aprile",
"given": "Pierangelo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cricelli",
"given": "Claudio",
"sequence": "additional"
}
],
"container-title": "Internal and Emergency Medicine",
"container-title-short": "Intern Emerg Med",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2022,
7,
30
]
],
"date-time": "2022-07-30T19:02:53Z",
"timestamp": 1659207773000
},
"deposited": {
"date-parts": [
[
2022,
8,
9
]
],
"date-time": "2022-08-09T17:19:46Z",
"timestamp": 1660065586000
},
"funder": [
{
"name": "Italian College of General Practitioners and Primary Care"
}
],
"indexed": {
"date-parts": [
[
2022,
8,
9
]
],
"date-time": "2022-08-09T17:41:59Z",
"timestamp": 1660066919325
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
7,
30
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.springer.com/tdm",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
7,
30
]
],
"date-time": "2022-07-30T00:00:00Z",
"timestamp": 1659139200000
}
},
{
"URL": "https://www.springer.com/tdm",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
7,
30
]
],
"date-time": "2022-07-30T00:00:00Z",
"timestamp": 1659139200000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1007/s11739-022-03054-1.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1007/s11739-022-03054-1/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1007/s11739-022-03054-1.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1007",
"published": {
"date-parts": [
[
2022,
7,
30
]
]
},
"published-online": {
"date-parts": [
[
2022,
7,
30
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1371/JOURNAL.PMED.1003777",
"author": "J Elliott",
"doi-asserted-by": "publisher",
"first-page": "e1003777",
"journal-title": "PLOS Med",
"key": "3054_CR1",
"unstructured": "Elliott J, Whitaker M, Bodinier B et al (2021) Predictive symptoms for COVID-19 in the community: REACT-1 study of over 1 million people. PLOS Med 18:e1003777. https://doi.org/10.1371/JOURNAL.PMED.1003777",
"volume": "18",
"year": "2021"
},
{
"DOI": "10.1002/14651858.CD013705.PUB2",
"author": "J Dinnes",
"doi-asserted-by": "publisher",
"journal-title": "Cochrane Database Syst Rev",
"key": "3054_CR2",
"unstructured": "Dinnes J, Deeks JJ, Berhane S et al (2021) Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD013705.PUB2",
"year": "2021"
},
{
"key": "3054_CR3",
"unstructured": "Home care for patients with suspected or confirmed COVID-19 and management of their contacts. https://www.who.int/publications/i/item/home-care-for-patients-with-suspected-novel-coronavirus-(ncov)-infection-presenting-with-mild-symptoms-and-management-of-contacts. Accessed 21 Feb 2022"
},
{
"key": "3054_CR4",
"unstructured": "NICE Overview | COVID-19 rapid guideline: managing COVID-19 | Guidance | NICE. https://www.nice.org.uk/guidance/ng191. Accessed 20 Feb 2022"
},
{
"key": "3054_CR5",
"unstructured": "CDC Treatments Your Healthcare Provider Might Recommend if You Are Sick | CDC. https://www.cdc.gov/coronavirus/2019-ncov/your-health/treatments-for-severe-illness.html. Accessed 20 Feb 2022"
},
{
"key": "3054_CR6",
"unstructured": "AIFA Medicines usable for treatment of COVID-19 disease | Italian Medicines Agency. https://www.aifa.gov.it/en/aggiornamento-sui-farmaci-utilizzabili-per-il-trattamento-della-malattia-covid19. Accessed 22 Feb 2022"
},
{
"DOI": "10.3390/IDR13010028",
"author": "DG DrI",
"doi-asserted-by": "publisher",
"journal-title": "Infect Dis Rep",
"key": "3054_CR7",
"unstructured": "DrI DG, A R et al (2021) How to treat COVID-19 patients at home in the Italian context: an expert opinion. Infect Dis Rep. https://doi.org/10.3390/IDR13010028",
"year": "2021"
},
{
"DOI": "10.1007/S40264-020-00965-W",
"author": "M Tuccori",
"doi-asserted-by": "publisher",
"first-page": "699",
"journal-title": "Drug Saf",
"key": "3054_CR8",
"unstructured": "Tuccori M, Convertino I, Ferraro S et al (2020) The impact of the COVID-19 “Infodemic” on drug-utilization behaviors: implications for pharmacovigilance. Drug Saf 43:699–709. https://doi.org/10.1007/S40264-020-00965-W",
"volume": "43",
"year": "2020"
},
{
"DOI": "10.1002/pds.5324",
"author": "S Enners",
"doi-asserted-by": "publisher",
"first-page": "1493",
"journal-title": "Pharmacoepidemiol Drug Saf",
"key": "3054_CR9",
"unstructured": "Enners S, Gradl G, Kieble M et al (2021) Utilization of drugs with reports on potential efficacy or harm on COVID-19 before, during, and after the first pandemic wave. Pharmacoepidemiol Drug Saf 30:1493–1503. https://doi.org/10.1002/pds.5324",
"volume": "30",
"year": "2021"
},
{
"DOI": "10.1002/jmv.27158",
"author": "S Pandolfi",
"doi-asserted-by": "publisher",
"first-page": "5704",
"journal-title": "J Med Virol",
"key": "3054_CR10",
"unstructured": "Pandolfi S, Simonetti V, Ricevuti G, Chirumbolo S (2021) Paracetamol in the home treatment of early COVID-19 symptoms: a possible foe rather than a friend for elderly patients? J Med Virol 93:5704–5706",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1016/j.eclinm.2021.100941",
"author": "F Suter",
"doi-asserted-by": "publisher",
"journal-title": "EClinicalMedicine",
"key": "3054_CR11",
"unstructured": "Suter F, Consolaro E, Pedroni S et al (2021) A simple, home-therapy algorithm to prevent hospitalisation for COVID-19 patients: a retrospective observational matched-cohort study. EClinicalMedicine 37:100941",
"volume": "37",
"year": "2021"
},
{
"DOI": "10.1016/J.BIOCEL.2005.03.005",
"author": "S Dimova",
"doi-asserted-by": "publisher",
"first-page": "1727",
"journal-title": "Int J Biochem Cell Biol",
"key": "3054_CR12",
"unstructured": "Dimova S, Hoet PHM, Dinsdale D, Nemery B (2005) Acetaminophen decreases intracellular glutathione levels and modulates cytokine production in human alveolar macrophages and type II pneumocytes in vitro. Int J Biochem Cell Biol 37:1727–1737. https://doi.org/10.1016/J.BIOCEL.2005.03.005",
"volume": "37",
"year": "2005"
},
{
"DOI": "10.1111/bcpt.13690",
"author": "S Pandolfi",
"doi-asserted-by": "publisher",
"first-page": "225",
"journal-title": "Basic Clin Pharmacol Toxicol",
"key": "3054_CR13",
"unstructured": "Pandolfi S, Chirumbolo S, Ricevuti G et al (2022) Home pharmacological therapy in early COVID-19 to prevent hospitalization and reduce mortality: time for a suitable proposal. Basic Clin Pharmacol Toxicol 130:225–239. https://doi.org/10.1111/bcpt.13690",
"volume": "130",
"year": "2022"
},
{
"key": "3054_CR14",
"unstructured": "Battaggia A Un algoritmo terapeutico domiciliare per prevenire i ricoveri? I risultati possono essere dovuti a sbilanciamento e a errore random - E&P Repository. https://repo.epiprev.it/index.php/download/un-algoritmo-terapeutico-domiciliare-per-prevenire-i-ricoveri-i-risultati-possono-essere-dovuti-a-sbilanciamento-e-a-errore-random/. Accessed 21 Feb 2022"
},
{
"author": "L Prada",
"key": "3054_CR15",
"unstructured": "Prada L, Santos DC, Baião RA et al (2021) Risk of SARS-CoV-2 Infection and COVID-19 Severity Associated With Exposure to Nonsteroidal Anti-Inflammatory Drugs: Systematic Review and Meta-Analysis. J. Clin, Pharmacol",
"volume-title": "Risk of SARS-CoV-2 Infection and COVID-19 Severity Associated With Exposure to Nonsteroidal Anti-Inflammatory Drugs: Systematic Review and Meta-Analysis",
"year": "2021"
},
{
"DOI": "10.1111/BCP.12705",
"author": "JL Faillie",
"doi-asserted-by": "publisher",
"first-page": "779",
"journal-title": "Br J Clin Pharmacol",
"key": "3054_CR16",
"unstructured": "Faillie JL (2015) Indication bias or protopathic bias? Br J Clin Pharmacol 80:779–780. https://doi.org/10.1111/BCP.12705",
"volume": "80",
"year": "2015"
},
{
"DOI": "10.1002/PDS.1360",
"author": "H Tamim",
"doi-asserted-by": "publisher",
"first-page": "250",
"journal-title": "Pharmacoepidemiol Drug Saf",
"key": "3054_CR17",
"unstructured": "Tamim H, Tahami Monfared AA, LeLorier J (2007) Application of lag-time into exposure definitions to control for protopathic bias. Pharmacoepidemiol Drug Saf 16:250–258. https://doi.org/10.1002/PDS.1360",
"volume": "16",
"year": "2007"
},
{
"DOI": "10.1111/BCP.14514",
"author": "R Vaja",
"doi-asserted-by": "publisher",
"first-page": "776",
"journal-title": "Br J Clin Pharmacol",
"key": "3054_CR18",
"unstructured": "Vaja R, Chan JSK, Ferreira P et al (2021) The COVID-19 ibuprofen controversy: a systematic review of NSAIDs in adult acute lower respiratory tract infections. Br J Clin Pharmacol 87:776–784. https://doi.org/10.1111/BCP.14514",
"volume": "87",
"year": "2021"
},
{
"DOI": "10.1093/pubmed/21.3.299",
"author": "R Lawrenson",
"doi-asserted-by": "publisher",
"first-page": "299",
"journal-title": "J Public Health Med",
"key": "3054_CR19",
"unstructured": "Lawrenson R, Williams T, Farmer R (1999) Clinical information for research; the use of general practice databases. J Public Health Med 21:299–304",
"volume": "21",
"year": "1999"
},
{
"key": "3054_CR20",
"unstructured": "Bianchini E, Brignoli O, Cricelli C VIII Report Health Search. Available at: http://healthsearch.it/documenti/Archivio/Report/VIIIReport_2013-2014/index.html."
},
{
"DOI": "10.1080/14760584.2022.2019582",
"author": "F Lapi",
"doi-asserted-by": "publisher",
"journal-title": "Expert Rev Vaccines",
"key": "3054_CR21",
"unstructured": "Lapi F, Domnich A, Marconi E et al (2021) Predicting the risk of severe COVID-19 outcomes in primary care: development and validation of a vulnerability index for equitable allocation of effective vaccines. Expert Rev Vaccines. https://doi.org/10.1080/14760584.2022.2019582",
"year": "2021"
},
{
"DOI": "10.1016/j.bone.2011.09.048",
"author": "F Lapi",
"doi-asserted-by": "publisher",
"journal-title": "Bone",
"key": "3054_CR22",
"unstructured": "Lapi F, Simonetti M, Michieli R et al (2012) Assessing 5-year incidence rates and determinants of osteoporotic fractures in primary care. Bone. https://doi.org/10.1016/j.bone.2011.09.048",
"year": "2012"
},
{
"DOI": "10.1016/j.ijcard.2016.08.269",
"author": "V Guglielmi",
"doi-asserted-by": "publisher",
"key": "3054_CR23",
"unstructured": "Guglielmi V, Bellia A, Pecchioli S et al (2016) What is the actual epidemiology of familial hypercholesterolemia in Italy? Int J Cardiol, Evidence from a National Primary Care Database. https://doi.org/10.1016/j.ijcard.2016.08.269",
"volume-title": "What is the actual epidemiology of familial hypercholesterolemia in Italy?",
"year": "2016"
},
{
"DOI": "10.1093/pubmed/fdg060",
"author": "C Cricelli",
"doi-asserted-by": "publisher",
"first-page": "254",
"journal-title": "J Pub Health Med",
"key": "3054_CR24",
"unstructured": "Cricelli C, Mazzaglia G, Samani F et al (2003) Prevalence estimates for chronic diseases in Italy: exploring the differences between self-report and primary care databases. J Pub Health Med 25:254–257",
"volume": "25",
"year": "2003"
},
{
"DOI": "10.1080/01621459.1996.10476660",
"author": "NE Breslow",
"doi-asserted-by": "publisher",
"journal-title": "J Am Stat Assoc",
"key": "3054_CR25",
"unstructured": "Breslow NE (1996) Statistics in epidemiology: the case-control study. J Am Stat Assoc. https://doi.org/10.1080/01621459.1996.10476660",
"year": "1996"
},
{
"DOI": "10.1016/j.jclinepi.2014.11.010",
"author": "GS Collins",
"doi-asserted-by": "publisher",
"first-page": "134",
"journal-title": "J Clin Epidemiol",
"key": "3054_CR26",
"unstructured": "Collins GS, Reitsma JB, Altman DG, Moons KGM (2015) Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. J Clin Epidemiol 68:134–143. https://doi.org/10.1016/j.jclinepi.2014.11.010",
"volume": "68",
"year": "2015"
},
{
"DOI": "10.1177/1536867X0800800103",
"author": "N Orsini",
"doi-asserted-by": "publisher",
"first-page": "29",
"journal-title": "Stata J",
"key": "3054_CR27",
"unstructured": "Orsini N, Bellocco R, Bottai M et al (2008) A tool for deterministic and probabilistic sensitivity analysis of epidemiologic studies. Stata J 8:29–48",
"volume": "8",
"year": "2008"
},
{
"DOI": "10.1002/pds.5346",
"author": "M Hempenius",
"doi-asserted-by": "publisher",
"first-page": "1703",
"journal-title": "Pharmacoepidemiol Drug Saf",
"key": "3054_CR28",
"unstructured": "Hempenius M, Groenwold RHH, de Boer A et al (2021) Drug exposure misclassification in pharmacoepidemiology: sources and relative impact. Pharmacoepidemiol Drug Saf 30:1703–1715. https://doi.org/10.1002/pds.5346",
"volume": "30",
"year": "2021"
},
{
"DOI": "10.1016/J.ECLINM.2021.100941",
"author": "F Suter",
"doi-asserted-by": "publisher",
"journal-title": "EClinicalMedicine",
"key": "3054_CR29",
"unstructured": "Suter F, Consolaro E, Pedroni S et al (2021) A simple, home-therapy algorithm to prevent hospitalisation for COVID-19 patients: a retrospective observational matched-cohort study. EClinicalMedicine. https://doi.org/10.1016/J.ECLINM.2021.100941",
"year": "2021"
},
{
"DOI": "10.1111/bcpt.13650",
"author": "S Pandolfi",
"doi-asserted-by": "publisher",
"first-page": "395",
"journal-title": "Basic Clin Pharmacol Toxicol",
"key": "3054_CR30",
"unstructured": "Pandolfi S, Chirumbolo S (2021) Home therapy of COVID-19 at the earliest may greatly prevent hospitalization. Basic Clin Pharmacol Toxicol 129:395–396. https://doi.org/10.1111/bcpt.13650",
"volume": "129",
"year": "2021"
},
{
"DOI": "10.1016/S0016-5085(75)80025-4",
"author": "JR Mitchell",
"doi-asserted-by": "publisher",
"first-page": "392",
"journal-title": "Gastroenterology",
"key": "3054_CR31",
"unstructured": "Mitchell JR, Jollows DJ (1975) Metabolic activation of drugs to toxic substances. Gastroenterology 68:392–410. https://doi.org/10.1016/S0016-5085(75)80025-4",
"volume": "68",
"year": "1975"
},
{
"author": "S Kennon-McGill",
"first-page": "297",
"journal-title": "J Clin Transl Res",
"key": "3054_CR32",
"unstructured": "Kennon-McGill S, McGill MR (2018) Extrahepatic toxicity of acetaminophen: critical evaluation of the evidence and proposed mechanisms. J Clin Transl Res 3:297–310",
"volume": "3",
"year": "2018"
},
{
"DOI": "10.1016/j.joca.2016.01.008",
"author": "J Ranstam",
"doi-asserted-by": "publisher",
"first-page": "763",
"journal-title": "Osteoarthr Cartil",
"key": "3054_CR33",
"unstructured": "Ranstam J (2016) Multiple P-values and Bonferroni correction. Osteoarthr Cartil 24:763–764. https://doi.org/10.1016/j.joca.2016.01.008",
"volume": "24",
"year": "2016"
},
{
"DOI": "10.1002/pds.1200",
"author": "S Schneeweiss",
"doi-asserted-by": "publisher",
"first-page": "291",
"journal-title": "Pharmacoepidemiol Drug Saf",
"key": "3054_CR34",
"unstructured": "Schneeweiss S (2006) Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf 15:291–303. https://doi.org/10.1002/pds.1200",
"volume": "15",
"year": "2006"
},
{
"DOI": "10.1056/NEJMC2119270/SUPPL_FILE/NEJMC2119270_DISCLOSURES.PDF",
"author": "S Collie",
"doi-asserted-by": "publisher",
"first-page": "494",
"journal-title": "N Engl J Med",
"key": "3054_CR35",
"unstructured": "Collie S, Champion J, Moultrie H et al (2022) Effectiveness of BNT162b2 vaccine against omicron variant in South Africa. N Engl J Med 386:494–496. https://doi.org/10.1056/NEJMC2119270/SUPPL_FILE/NEJMC2119270_DISCLOSURES.PDF",
"volume": "386",
"year": "2022"
}
],
"reference-count": 35,
"references-count": 35,
"relation": {},
"resource": {
"primary": {
"URL": "https://link.springer.com/10.1007/s11739-022-03054-1"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Emergency Medicine",
"Internal Medicine"
],
"subtitle": [],
"title": "To clarify the safety profile of paracetamol for home-care patients with COVID-19: a real-world cohort study, with nested case–control analysis, in primary care",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy"
}