COVID-19 convalescent plasma to treat hospitalised COVID-19 patients with or without underlying immunodeficiency
et al., medRxiv, doi:10.1101/2022.08.09.22278329, CORIPLASM, NCT04345991, Aug 2022
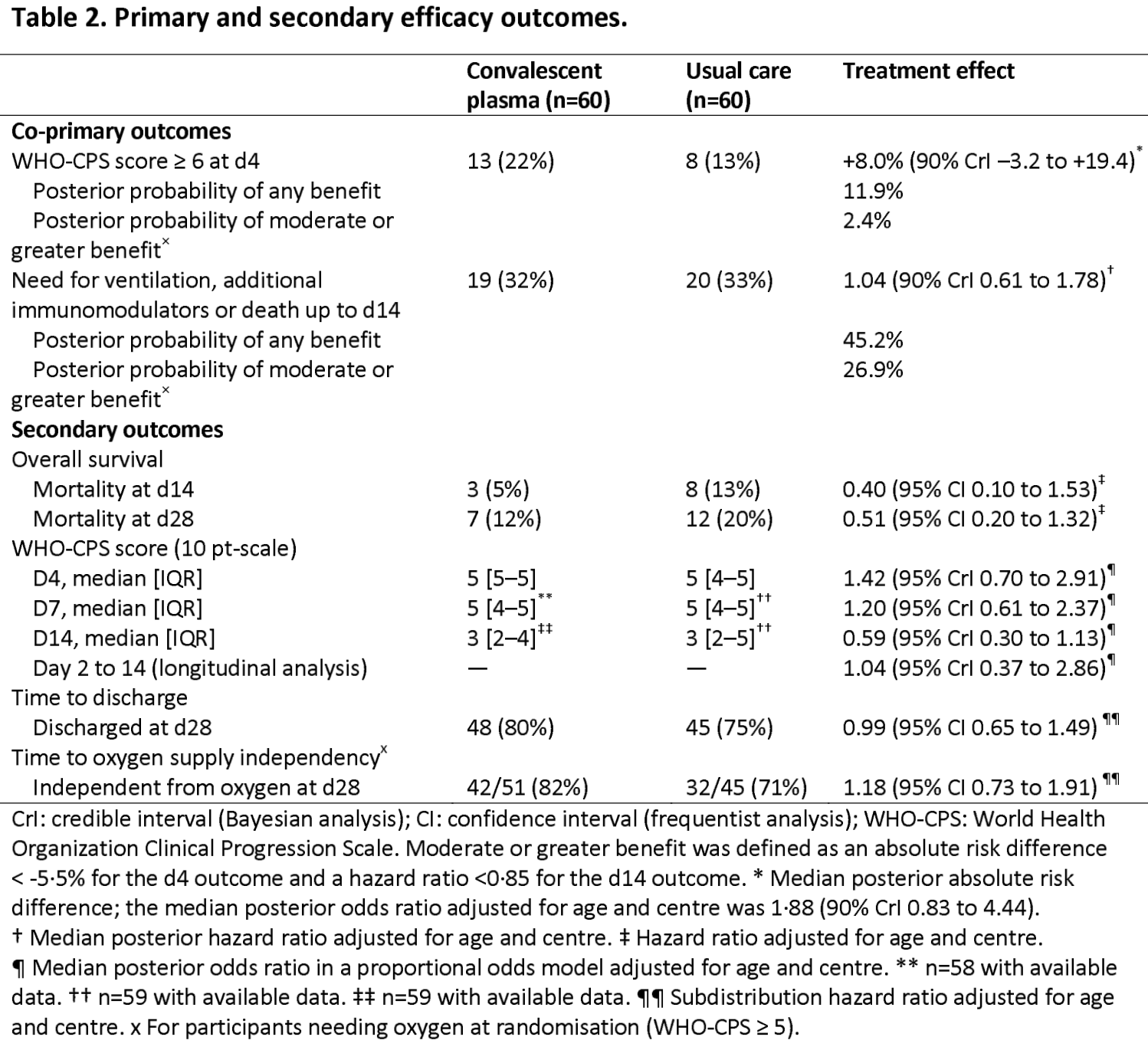
RCT 120 hospitalized patients in France, showing no significant difference in outcomes with convalescent plasma treatment, with the exception of lower mortality in the subgroup of immunosuppressed patients.
|
risk of death, 49.0% lower, HR 0.51, p = 0.16, treatment 7 of 60 (11.7%), control 12 of 60 (20.0%), NNT 12, adjusted per study, day 28.
|
|
risk of death, 64.0% lower, HR 0.36, p = 0.04, treatment 4 of 22 (18.2%), control 11 of 27 (40.7%), NNT 4.4, adjusted per study, day 28, immunocompromised.
|
|
risk of progression, 68.3% higher, RR 1.68, p = 0.18, treatment 13 of 60 (21.7%), control 8 of 60 (13.3%), adjusted per study, odds ratio converted to relative risk, WHO-CPS ≥6, day 4, primary outcome.
|
|
risk of progression, 4.0% higher, HR 1.04, p = 0.89, treatment 19 of 60 (31.7%), control 20 of 60 (33.3%), NNT 60, adjusted per study, ventilation, additional immunomodulators, or death, day 14, primary outcome.
|
|
hospitalization time, 6.7% higher, relative time 1.07, p = 0.99, treatment 60, control 60.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Lacombe et al., 10 Aug 2022, Randomized Controlled Trial, France, preprint, 33 authors, study period 16 April, 2020 - 21 April, 2021, average treatment delay 7.0 days, trial NCT04345991 (history) (CORIPLASM).
Contact: karine.lacombe2@aphp.fr, raphael.porcher@aphp.fr.
COVID-19 convalescent plasma to treat hospitalised COVID-19 patients with or without underlying immunodeficiency
doi:10.1101/2022.08.09.22278329
The authors wish to thank all physicians, nurses, and assistant nurses who took care of the patients, clinical research assistants and clinical research doctors who included and followed the patients during the trial, physicians, nurses and staff involved in convalescent plasma collection, manufacturing, testing and issuing, and above all the patients who agreed to participate in the study and the convalescent donors who generously gave their plasma. Special thanks to the DRCI of Assistance Publique-Hôpitaux de Paris (APHP), the trial sponsor, and the Unité de Recherche de l'Est Parisien (URC-EST, APHP.SU, site St Antoine site), which managed the trial.
Role of the funding source The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. Contributors KL and PT were involved in the protocol design and study design, including conceptualization, methodology, funding acquisition, and resources. NS and TS did the data curation, investigation,
References
Avendaño-Solá, Ramos-Martínez, Muñez-Rubio, A multicenter randomized open-label clinical trial for convalescent plasma in patients hospitalized with COVID-19 pneumonia, J Clin Invest
Bar, Shaw, Choi, Aqui, A randomized controlled study of convalescent plasma for individuals hospitalized with COVID-19 pneumonia, J Clin Invest
Beigel, Tomashek, Dodd, Remdesivir for the Treatment of Covid-19 -Final Report, N Engl J Med
Bernal, Da Silva, Musungaie, Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients, N Engl J Med
Cao, Yisimay, Jian, Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies, Nature, doi:10.1038/s41586-021-04385-3
Casadevall, Pirofski, The convalescent sera option for containing COVID-19, J Clin Invest
Duléry, Lamure, Delord, Prolonged in-hospital stay and higher mortality after Covid-19 among patients with non-Hodgkin lymphoma treated with B-cell depleting immunotherapy, Am J Hematol
Estcourt, Cohn, Pagano, Clinical Practice Guidelines From the Association for the Advancement of Blood and Biotherapies (AABB): COVID-19 Convalescent Plasma, Ann Intern Med
Estcourt, Turgeon, Mcquilten, Effect of Convalescent Plasma on Organ Support-Free Days in Critically Ill Patients With COVID-19: A Randomized Clinical Trial, JAMA
Gallian, Pastorino, Morel, Lower prevalence of antibodies neutralizing SARS-CoV-2 in group O French blood donors, Antiviral Res
Hammond, Leister-Tebbe, Gardner, Oral Nirmatrelvir for High-Risk, Non hospitalized Adults with Covid-19, N Engl J Med
Hermine, Tharaux, Effect of Tocilizumab vs Usual Care in Adults Hospitalized With COVID-19 and Moderate or Severe Pneumonia: A Randomized Clinical Trial, JAMA Intern Med
Hueso, Godron, Lanoy, Convalescent plasma improves overall survival in patients with B-cell lymphoid malignancy and COVID-19: a longitudinal cohort and propensity score analysis, Leukemia
Janiaud, Axfors, Schmitt, Association of Convalescent Plasma Treatment With Clinical outcomes in Patients With COVID-19: A Systematic Review and Meta-analysis, JAMA
Joyner, Carter, Senefeld, Convalescent Plasma Antibody Levels and the Risk of Death from Covid-19, N Engl J Med
Junqueira, Crespo, Ranjbar, FcγR-mediated SARS-CoV-2 infection of monocytes activates inflammation, Nature
Körper, Weiss, Zickler, Results of the CAPSID randomized trial for high-dose convalescent plasma in patients with severe COVID-19, J Clin Invest
Lee, Wheatley, Kent, Dekosky, Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies, Nat Microbiol
Li, Beck, Laeyendecker, Eby, Convalescent plasma with a high level of virus-specific antibody effectively neutralizes SARS-CoV-2 variants of concern, Blood Adv
Libster, Marc, Wappner, Early High-Titer Plasma Therapy to Prevent Severe Covid-19 in Older Adults, N Engl J Med
Male, None, n/N (%
O'donnell, Grinsztejn, Cummings, A randomized double-blind controlled trial of convalescent plasma in adults with severe COVID-19, J Clin Invest
Persad, Peek, Shah, Fair Allocation of Scarce Therapies for COVID-19, Clin Infect Di
Piechotta, Iannizzi, Chai, Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review, Cochrane Database Syst Rev
Planas, Saunders, Maes, Considerable escape of SARS-CoV-2 Omicron to antibody neutralization, Nature
Rössler, Riepler, Bante, Laer, Kimpel, SARS-CoV-2 Omicron Variant Neutralization in Serum from Vaccinated and Convalescent Persons, N Engl J Med
Sefik, Qu, Junqueira, Kaffe, Inflammasome activation in infected macrophages drives COVID-19 pathology, Nature
Stamatatos, Czartoski, Wan, mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection, Science
Sullivan, Gebo, Shoham, Early Outpatient Treatment for Covid-19 with Convalescent Plasma, N Engl J Med
Thompson, Henderson, Shah, Association of Convalescent Plasma Therapy With Survival in Patients With Hematologic Cancers and COVID-19, JAMA Oncol
Weinreich, Sivapalasingam, Norton, REGEN-COV Antibody Combination and Outcomes in Outpatients with Covid-19, N Engl J Med
DOI record:
{
"DOI": "10.1101/2022.08.09.22278329",
"URL": "http://dx.doi.org/10.1101/2022.08.09.22278329",
"abstract": "<jats:p>Background: Efficacy of COVID-19 convalescent plasma (CCP) in COVID-19 pneumonia is uncertain. Early transfusion of high antibody titre CCP may be beneficial, especially in case of underlying immunosuppression. Methods: The CORIPLASM study was a multicentric, open-label, Bayesian randomised clinical trial evaluating the efficacy of CCP in patients with moderate COVID-19 pneumonia, including patients with underlying immunosuppression. Patients hospitalised with COVID-19 for less than 9 days were assigned to receive 2 plasma units/day over 2 days (CCP) or usual care (UC) alone. Primary outcomes were the proportion of patients with a WHO-Clinical Progression Score (CPS) >= 6 on the 10-point scale on day 4 and survival without ventilation or additional immunomodulatory treatment by day 14. Main analysis was conducted on the whole population and a planned subgroup analysis was performed according to immunosuppression status. Findings: A total of 120 patients were recruited between April 16, 2020, and April 21, 2021, and assigned to CCP (n=60) or UC (n=60) with a 28 day-follow-up. The median time from symptoms onset to randomisation (days) was 7.0 [interquartile range (IQR) 5.0-9.0] and 7.0 [IQR 4.0-8.5] in CCP and UC, respectively. Thirteen (22%) patients with CCP had a WHO-CPS >= 6 at day 4 versus 8 (13%) with UC, adjusted odds ratio (aOR) 1.88 [95% confidence interval (CI), 0.71 to 5.24]. By d14, 19 (31.6%) patients with CCP and 20 (33.3%) patients with UC had ventilation, additional immunomodulatory treatment or had died. Cumulative incidence of death was 3 (5%) with CCP and 8 (13%) with UC at d14 (aHR 0.40 [95%CI 0.10 -1.53]), and 7 (12%) with CCP and 12 (20%) with UC at day 28 (aHR 0.51 [95% CI 0.20-1.32]). Subgroup analysis indicated that CCP might be associated with a lower mortality in patients with underlying immunosuppression (HR 0.37 [95% CI 0.14-0.97]). Serious adverse events were noted in 30 (50%) and 26 (43%) patients with CCP or UC, respectively. Interpretation: CCP treatment did not improve early outcomes in patients with mild-to-moderate form COVID-19 pneumonia but was associated with reduced mortality in the subgroup of immunosuppressed patients. Trial registration: clinicaltrials.gov Identifier:<jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"clintrialgov\" xlink:href=\"NCT04345991\">NCT04345991</jats:ext-link></jats:p>",
"accepted": {
"date-parts": [
[
2022,
10,
27
]
]
},
"author": [
{
"affiliation": [],
"family": "Lacombe",
"given": "Karine",
"sequence": "first"
},
{
"affiliation": [],
"family": "Hueso",
"given": "Thomas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Porcher",
"given": "Raphael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mekinian",
"given": "Arsene",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chiarabini",
"given": "Thibault",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6668-8854",
"affiliation": [],
"authenticated-orcid": false,
"family": "Georgin-Lavialle",
"given": "Sophie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ader",
"given": "Florence",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saison",
"given": "Julien",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Martin-Blondet",
"given": "Guillaume",
"sequence": "additional"
},
{
"affiliation": [],
"family": "De Castro",
"given": "Nathalie A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bonnet",
"given": "Fabrice",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cazanave",
"given": "Charles",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Francois",
"given": "Anne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Morel",
"given": "Pascal",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2574-3874",
"affiliation": [],
"authenticated-orcid": false,
"family": "Hermine",
"given": "Olivier",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pourcher",
"given": "Valerie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Michel",
"given": "Marc",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lescure",
"given": "Xavier",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Soussi",
"given": "Nora",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brun",
"given": "Phillipe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pommeret",
"given": "Fanny",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sellier",
"given": "Pierre",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rousset",
"given": "Stella",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Piroth",
"given": "Lione",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3482-3331",
"affiliation": [],
"authenticated-orcid": false,
"family": "Michot",
"given": "Jean-Marie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "baron",
"given": "gabriel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "de Lamballerie",
"given": "Xavier",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mariette",
"given": "Xavier",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6062-5905",
"affiliation": [],
"authenticated-orcid": false,
"family": "Tharaux",
"given": "Pierre-Louis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Resche-Rigaux",
"given": "Matthieu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ravaud",
"given": "Philippe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Simon",
"given": "Tabassome",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9310-8322",
"affiliation": [],
"authenticated-orcid": false,
"family": "Tiberghien",
"given": "Pierre",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
8,
10
]
],
"date-time": "2022-08-10T17:20:19Z",
"timestamp": 1660152019000
},
"deposited": {
"date-parts": [
[
2022,
10,
27
]
],
"date-time": "2022-10-27T22:55:11Z",
"timestamp": 1666911311000
},
"group-title": "Infectious Diseases (except HIV/AIDS)",
"indexed": {
"date-parts": [
[
2022,
10,
28
]
],
"date-time": "2022-10-28T04:54:59Z",
"timestamp": 1666932899500
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
8,
10
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2022.08.09.22278329",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2022,
8,
10
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2022,
8,
10
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference": [
{
"DOI": "10.1172/JCI138003",
"article-title": "The convalescent sera option for containing COVID-19",
"doi-asserted-by": "crossref",
"first-page": "1545",
"issue": "4",
"journal-title": "J Clin Invest",
"key": "2022081205500804000_2022.08.09.22278329v1.1",
"volume": "130",
"year": "2020"
},
{
"DOI": "10.1002/14651858.CD013600.pub4",
"doi-asserted-by": "publisher",
"key": "2022081205500804000_2022.08.09.22278329v1.2"
},
{
"DOI": "10.1056/NEJMoa2033700",
"doi-asserted-by": "publisher",
"key": "2022081205500804000_2022.08.09.22278329v1.3"
},
{
"DOI": "10.1056/NEJMoa2119657",
"article-title": "Early Outpatient Treatment for Covid-19 with Convalescent Plasma",
"doi-asserted-by": "crossref",
"first-page": "1700",
"journal-title": "N Engl J Med",
"key": "2022081205500804000_2022.08.09.22278329v1.4",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1001/jamaoncol.2021.1799",
"article-title": "Association of Convalescent Plasma Therapy With Survival in Patients With Hematologic Cancers and COVID-19",
"doi-asserted-by": "crossref",
"first-page": "1167",
"journal-title": "JAMA Oncol",
"key": "2022081205500804000_2022.08.09.22278329v1.5",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.1038/s41375-022-01511-6",
"doi-asserted-by": "publisher",
"key": "2022081205500804000_2022.08.09.22278329v1.6"
},
{
"DOI": "10.1056/nejmoa2108163",
"doi-asserted-by": "publisher",
"key": "2022081205500804000_2022.08.09.22278329v1.7"
},
{
"DOI": "10.1016/S0140-6736(22)00163-5",
"doi-asserted-by": "publisher",
"key": "2022081205500804000_2022.08.09.22278329v1.8"
},
{
"DOI": "10.1093/cid/ciab1039",
"doi-asserted-by": "crossref",
"key": "2022081205500804000_2022.08.09.22278329v1.9",
"unstructured": "Persad G , Peek ME , Shah SK. Fair Allocation of Scarce Therapies for COVID-19. Clin Infect Di. 2021; ciab1039."
},
{
"DOI": "10.1101/2021.05.26.445838",
"doi-asserted-by": "publisher",
"key": "2022081205500804000_2022.08.09.22278329v1.10"
},
{
"DOI": "10.1038/s41586-021-04389-z",
"article-title": "Considerable escape of SARS-CoV-2 Omicron to antibody neutralization",
"doi-asserted-by": "crossref",
"first-page": "671",
"journal-title": "Nature",
"key": "2022081205500804000_2022.08.09.22278329v1.11",
"volume": "602",
"year": "2022"
},
{
"DOI": "10.1182/bloodadvances.2022007410",
"article-title": "Convalescent plasma with a high level of virus-specific antibody effectively neutralizes SARS-CoV-2 variants of concern",
"doi-asserted-by": "crossref",
"first-page": "3678",
"journal-title": "Blood Adv",
"key": "2022081205500804000_2022.08.09.22278329v1.12",
"volume": "6",
"year": "2022"
},
{
"DOI": "10.1056/NEJMc2119236",
"article-title": "SARS-CoV-2 Omicron Variant Neutralization in Serum from Vaccinated and Convalescent Persons",
"doi-asserted-by": "crossref",
"first-page": "698",
"journal-title": "N Engl J Med",
"key": "2022081205500804000_2022.08.09.22278329v1.13",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2116044",
"article-title": "Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients",
"doi-asserted-by": "crossref",
"first-page": "509",
"journal-title": "N Engl J Med",
"key": "2022081205500804000_2022.08.09.22278329v1.14",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/nejmoa2118542",
"doi-asserted-by": "publisher",
"key": "2022081205500804000_2022.08.09.22278329v1.15"
},
{
"DOI": "10.1056/NEJMoa2007764",
"doi-asserted-by": "publisher",
"key": "2022081205500804000_2022.08.09.22278329v1.16"
},
{
"DOI": "10.1001/jamainternmed.2020.6820",
"article-title": "Effect of Tocilizumab vs Usual Care in Adults Hospitalized With COVID-19 and Moderate or Severe Pneumonia: A Randomized Clinical Trial",
"doi-asserted-by": "crossref",
"first-page": "32",
"journal-title": "JAMA Intern Med",
"key": "2022081205500804000_2022.08.09.22278329v1.17",
"volume": "181",
"year": "2021"
},
{
"DOI": "10.1016/j.antiviral.2020.104880",
"doi-asserted-by": "publisher",
"key": "2022081205500804000_2022.08.09.22278329v1.18"
},
{
"DOI": "10.1001/jama.2021.2747",
"article-title": "Association of Convalescent Plasma Treatment With Clinical outcomes in Patients With COVID-19: A Systematic Review and Meta-analysis",
"doi-asserted-by": "crossref",
"first-page": "1185",
"journal-title": "JAMA",
"key": "2022081205500804000_2022.08.09.22278329v1.19",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1172/JCI150646",
"article-title": "A randomized double-blind controlled trial of convalescent plasma in adults with severe COVID-19",
"doi-asserted-by": "crossref",
"first-page": "e150646",
"journal-title": "J Clin Invest",
"key": "2022081205500804000_2022.08.09.22278329v1.20",
"volume": "131",
"year": "2021"
},
{
"DOI": "10.1172/JCI155114",
"doi-asserted-by": "publisher",
"key": "2022081205500804000_2022.08.09.22278329v1.21"
},
{
"DOI": "10.1001/jama.2021.18178",
"article-title": "Effect of Convalescent Plasma on Organ Support-Free Days in Critically Ill Patients With COVID-19: A Randomized Clinical Trial",
"author": "McQuilten",
"doi-asserted-by": "crossref",
"first-page": "1690",
"journal-title": "JAMA",
"key": "2022081205500804000_2022.08.09.22278329v1.22",
"volume": "326",
"year": "2021"
},
{
"DOI": "10.1002/ajh.26209",
"article-title": "Prolonged in-hospital stay and higher mortality after Covid-19 among patients with non-Hodgkin lymphoma treated with B-cell depleting immunotherapy",
"doi-asserted-by": "crossref",
"first-page": "934",
"journal-title": "Am J Hematol",
"key": "2022081205500804000_2022.08.09.22278329v1.23",
"volume": "96",
"year": "2021"
},
{
"DOI": "10.1172/JCI152264",
"article-title": "Results of the CAPSID randomized trial for high-dose convalescent plasma in patients with severe COVID-19",
"doi-asserted-by": "crossref",
"first-page": "e152264",
"journal-title": "J Clin Invest",
"key": "2022081205500804000_2022.08.09.22278329v1.24",
"volume": "131",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2031893",
"doi-asserted-by": "publisher",
"key": "2022081205500804000_2022.08.09.22278329v1.25"
},
{
"DOI": "10.1126/science.abg9175",
"doi-asserted-by": "publisher",
"key": "2022081205500804000_2022.08.09.22278329v1.26"
},
{
"DOI": "10.1172/JCI152740",
"doi-asserted-by": "publisher",
"key": "2022081205500804000_2022.08.09.22278329v1.27"
},
{
"DOI": "10.1038/s41564-020-00789-5",
"article-title": "Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies",
"doi-asserted-by": "crossref",
"first-page": "1185",
"journal-title": "Nat Microbiol",
"key": "2022081205500804000_2022.08.09.22278329v1.28",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1038/s41586-022-04702-4",
"article-title": "FcγR-mediated SARS-CoV-2 infection of monocytes activates inflammation",
"doi-asserted-by": "crossref",
"first-page": "576",
"journal-title": "Nature",
"key": "2022081205500804000_2022.08.09.22278329v1.29",
"volume": "606",
"year": "2022"
},
{
"DOI": "10.1038/s41586-022-04802-1",
"article-title": "Inflammasome activation in infected macrophages drives COVID-19 pathology",
"doi-asserted-by": "crossref",
"first-page": "585",
"journal-title": "Nature",
"key": "2022081205500804000_2022.08.09.22278329v1.30",
"volume": "606",
"year": "2022"
}
],
"reference-count": 30,
"references-count": 30,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2022.08.09.22278329"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "COVID-19 convalescent plasma to treat hospitalised COVID-19 patients with or without underlying immunodeficiency",
"type": "posted-content"
}