The influence of propolis plus Hyoscyamus niger L. against COVID‐19: A phase II, multicenter, placebo‐controlled, randomized trial
et al., Phytotherapy Research, doi:10.1002/ptr.8047, IRCT20200516047462N1, Nov 2023
RCT 140 patients showing lower progression and improved recovery with propolis plus Hyoscyamus niger L.syrup.
|
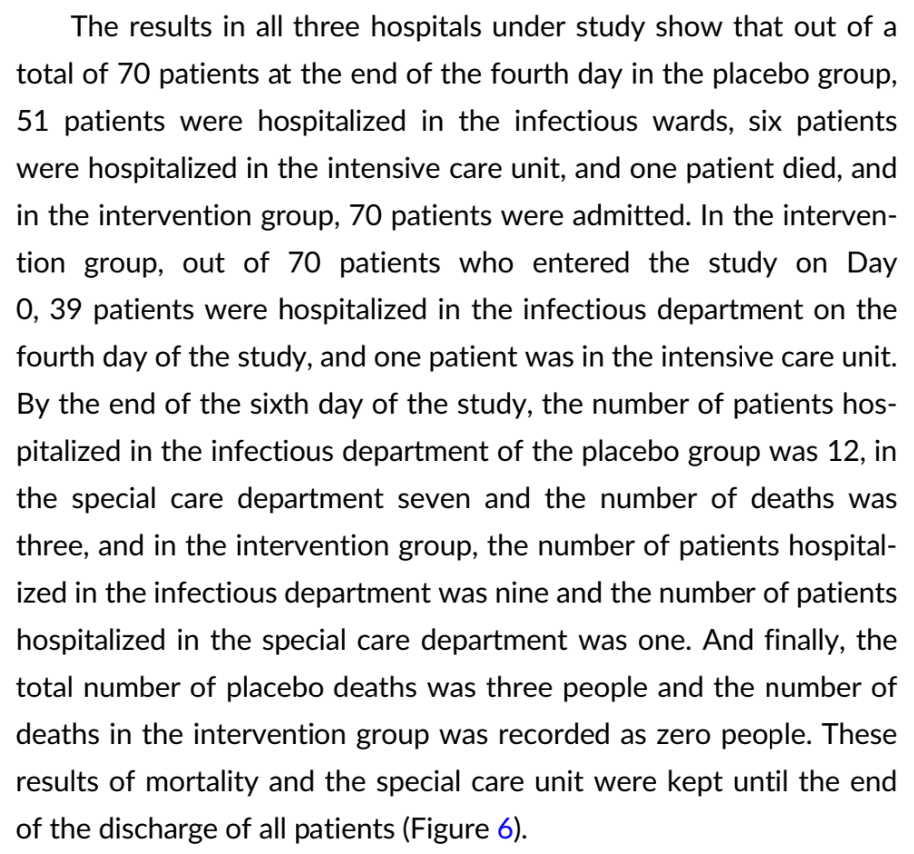
risk of death, 85.7% lower, RR 0.14, p = 0.24, treatment 0 of 70 (0.0%), control 3 of 70 (4.3%), NNT 23, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), day 6.
|
|
risk of ICU admission, 85.7% lower, RR 0.14, p = 0.06, treatment 1 of 70 (1.4%), control 7 of 70 (10.0%), NNT 12.
|
|
risk of no recovery, 63.6% lower, RR 0.36, p = 0.007, treatment 8 of 70 (11.4%), control 22 of 70 (31.4%), NNT 5.0, day 6.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Kosari et al., 22 Nov 2023, Double Blind Randomized Controlled Trial, placebo-controlled, Iran, peer-reviewed, 15 authors, this trial uses multiple treatments in the treatment arm (combined with Hyoscyamus niger L.) - results of individual treatments may vary, trial IRCT20200516047462N1.
Contact: banafshe57@hotmail.com.
DOI record:
{
"DOI": "10.1002/ptr.8047",
"ISSN": [
"0951-418X",
"1099-1573"
],
"URL": "http://dx.doi.org/10.1002/ptr.8047",
"abstract": "<jats:title>Abstract</jats:title><jats:p>The incubation period of COVID‐19 symptoms, along with the proliferation and high transmission rate of the SARS‐CoV‐2 virus, is the cause of an uncontrolled epidemic worldwide. Vaccination is the front line of prevention, and antiinflammatory and antiviral drugs are the treatment of this disease. In addition, some herbal therapy approaches can be a good way to deal with this disease. The aim of this study was to evaluate the effect of propolis syrup with <jats:italic>Hyoscyamus niger</jats:italic> L. extract in hospitalized patients with COVID‐19 with acute disease conditions in a double‐blinded approach. The study was performed on 140 patients with COVID‐19 in a double‐blind, randomized, and multicentral approach. The main inclusion criterion was the presence of a severe type of COVID‐19 disease. The duration of treatment with syrup was 6 days and 30 CC per day in the form of three meals. On Days 0, 2, 4, and 6, arterial blood oxygen levels, C‐reactive protein (CRP), erythrocyte sedimentation rate, and white blood cell, as well as the patient's clinical symptoms such as fever and chills, cough and shortness of breath, chest pain, and other symptoms, were recorded and analyzed. Propolis syrup with <jats:italic>H. niger</jats:italic> L. significantly reduces cough from the second day, relieving shortness of breath on the fourth day, and significantly reduces CRP, weakness, and lethargy, as well as significantly increased arterial blood oxygen pressure on the sixth day compared to the placebo group (<jats:italic>p</jats:italic> < 0.05). The results in patients are such that in the most severe conditions of the disease 80% < SpO<jats:sub>2</jats:sub> (oxygen saturation), the healing process of the syrup on reducing CRP and increasing arterial blood oxygen pressure from the fourth day is significantly different compared with the placebo group (<jats:italic>p</jats:italic> < 0.05). The use of syrup is associated with a reduction of 3.6 days in the hospitalization period compared with the placebo group. Propolis syrup with <jats:italic>H. niger</jats:italic> L. has effectiveness in the viral and inflammatory phases on clinical symptoms and blood parameters and arterial blood oxygen levels of patients with COVID‐19. Also, it reduces referrals to the intensive care unit and mortality in hospitalized patients with COVID‐19. So, this syrup promises to be an effective treatment in the great challenge of COVID‐19.</jats:p>",
"alternative-id": [
"10.1002/ptr.8047"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2022-10-20"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2023-08-16"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2023-11-22"
}
],
"author": [
{
"affiliation": [
{
"name": "Physiology Research Center, Institute for Basic Sciences Kashan University of Medical Sciences Kashan Iran"
},
{
"name": "Department of Biology, Science and Research Branch Islamic Azad University Tehran Iran"
}
],
"family": "Kosari",
"given": "Morteza",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Infectious Disease, Medical School Isfahan University of Medical Science Isfahan Iran"
},
{
"name": "Nosocomial Infection Research Center Isfahan University of Medical Sciences Isfahan Iran"
}
],
"family": "Khorvash",
"given": "Farzin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases Research Center Kashan University of Medical Sciences Kashan Iran"
}
],
"family": "Sayyah",
"given": "Mohammad Kazem",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, Infectious Diseases and Tropical Medicine Research Center Isfahan University of Medical Sciences Isfahan Iran"
}
],
"family": "Ansari Chaharsoughi",
"given": "Maryam",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases Research Center Kashan University of Medical Sciences Kashan Iran"
}
],
"family": "Najafi",
"given": "Ahmad",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases Research Center Kashan University of Medical Sciences Kashan Iran"
}
],
"family": "Momen‐Heravi",
"given": "Mansooreh",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2938-8902",
"affiliation": [
{
"name": "Department of Molecular and Cell Biology, Faculty of Basic Sciences University of Mazandaran Babolsar Iran"
}
],
"authenticated-orcid": false,
"family": "Karimian",
"given": "Mohammad",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Social Determinants of Health Research Center, Department of Biostatistics and Epidemiology, School of Public Health Kashan University of Medical Sciences Kashan Iran"
}
],
"family": "Akbari",
"given": "Hossein",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Physiology Research Center, Institute for Basic Sciences Kashan University of Medical Sciences Kashan Iran"
}
],
"family": "Noureddini",
"given": "Mehdi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Physiology Research Center, Institute for Basic Sciences Kashan University of Medical Sciences Kashan Iran"
}
],
"family": "Salami",
"given": "Mahmoud",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Addiction Studies, School of Medical, Clinical Research Development Unit‐Matini/Kargarnejad Hospital Kashan University of Medical Sciences Kashan Iran"
}
],
"family": "Ghaderi",
"given": "Amir",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Anatomical Research Center Kashan University of Medical Sciences Kashan Iran"
},
{
"name": "Sarem Fertility and Infertility Research Center, Sarem Women's Hospital Iran University of Medical Sciences (IUMS) Tehran Iran"
},
{
"name": "Sarem Cell Research Center Sarem Women's Hospital Tehran Iran"
}
],
"family": "Amini Mahabadi",
"given": "Javad",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Persian Medicine School of Persian Medicine, Tehran University of Medical Sciences Tehran Iran"
}
],
"family": "Khamechi",
"given": "Seyed Peyman",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Mathematical Sciences Isfahan University of Technology Isfahan Iran"
}
],
"family": "Yeganeh",
"given": "Somayeh",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4450-8861",
"affiliation": [
{
"name": "Physiology Research Center, Institute for Basic Sciences Kashan University of Medical Sciences Kashan Iran"
},
{
"name": "Department of Pharmacology, School of Medicine Kashan University of Medical Sciences Kashan Iran"
}
],
"authenticated-orcid": false,
"family": "Banafshe",
"given": "Hamid Reza",
"sequence": "additional"
}
],
"container-title": "Phytotherapy Research",
"container-title-short": "Phytotherapy Research",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2023,
11,
24
]
],
"date-time": "2023-11-24T16:23:39Z",
"timestamp": 1700843019000
},
"deposited": {
"date-parts": [
[
2024,
1,
21
]
],
"date-time": "2024-01-21T03:42:23Z",
"timestamp": 1705808543000
},
"indexed": {
"date-parts": [
[
2024,
1,
21
]
],
"date-time": "2024-01-21T05:05:29Z",
"timestamp": 1705813529148
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2023,
11,
22
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2024,
1
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://onlinelibrary.wiley.com/termsAndConditions#vor",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
11,
22
]
],
"date-time": "2023-11-22T00:00:00Z",
"timestamp": 1700611200000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/ptr.8047",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"page": "400-410",
"prefix": "10.1002",
"published": {
"date-parts": [
[
2023,
11,
22
]
]
},
"published-online": {
"date-parts": [
[
2023,
11,
22
]
]
},
"published-print": {
"date-parts": [
[
2024,
1
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.3390/molecules26051232",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_2_1"
},
{
"DOI": "10.15252/emmm.202013038",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_3_1"
},
{
"DOI": "10.3390/nu11112705",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_4_1"
},
{
"DOI": "10.1513/AnnalsATS.202008-940OC",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_5_1"
},
{
"DOI": "10.1038/s41598-022-05832-5",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_6_1"
},
{
"DOI": "10.1056/NEJMoa2001282",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_7_1"
},
{
"DOI": "10.1513/AnnalsATS.202005-478SD",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_8_1"
},
{
"DOI": "10.1038/s41467-021-27797-1",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_9_1"
},
{
"DOI": "10.12788/jhm.3560",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_10_1"
},
{
"DOI": "10.1128/AAC.01897-20",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_11_1"
},
{
"DOI": "10.1016/j.pharmthera.2020.107745",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_12_1"
},
{
"DOI": "10.1016/S0140-6736(21)02753-7",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_13_1"
},
{
"DOI": "10.1164/rccm.202009-3533SO",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_14_1"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_15_1"
},
{
"DOI": "10.1164/rccm.202005-1885OC",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_16_1"
},
{
"article-title": "Chemical compositions of essential oils, antimicrobial effect and antioxidant activity studies of Hyoscyamus niger L. from Turkey",
"author": "İnci Ş.",
"journal-title": "bioRxiv",
"key": "e_1_2_11_17_1",
"year": "2022"
},
{
"DOI": "10.3947/ic.2020.52.2.212",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_18_1"
},
{
"DOI": "10.1155/2016/4156293",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_19_1"
},
{
"DOI": "10.1007/s00705-022-05545-0",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_20_1"
},
{
"DOI": "10.1002/ptr.7116",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_21_1"
},
{
"DOI": "10.1038/s41467-022-28068-3",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_22_1"
},
{
"article-title": "Clinical spectrum of severe acute respiratory syndrome coronavirus 2 infection and protection from symptomatic reinfection",
"author": "Maier H. E.",
"journal-title": "Clinical Infectious Diseases",
"key": "e_1_2_11_23_1",
"volume": "75",
"year": "2021"
},
{
"DOI": "10.3389/fphar.2021.583387",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_24_1"
},
{
"DOI": "10.1016/j.jsbmb.2019.105573",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_25_1"
},
{
"article-title": "Effect of propolis on moderate persistent asthma: A phase two randomized, double blind, controlled clinical trial",
"author": "Mirsadraee M.",
"first-page": "22",
"journal-title": "Avicenna Journal of Phytomedicine",
"key": "e_1_2_11_26_1",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1002/rmv.2221",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_27_1"
},
{
"DOI": "10.1016/j.xcrm.2020.100144",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_28_1"
},
{
"DOI": "10.1038/s41590-017-0006-x",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_29_1"
},
{
"DOI": "10.1016/S0140-6736(21)02392-8",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_30_1"
},
{
"DOI": "10.1016/S0140-6736(21)01825-0",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_31_1"
},
{
"article-title": "Case report: C‐reactive protein apheresis in a patient with COVID‐19 and fulminant CRP increase",
"author": "Ringel J.",
"first-page": "3140",
"journal-title": "Frontiers in Immunology",
"key": "e_1_2_11_32_1",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1093/jpp/rgaa067",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_33_1"
},
{
"DOI": "10.1007/s11356-019-07458-z",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_34_1"
},
{
"article-title": "Efficacy of propolis as an adjunct treatment for hospitalized COVID‐19 patients: A randomized, controlled clinical trial",
"author": "Silveira M. A. D.",
"journal-title": "MedRxiv",
"key": "e_1_2_11_35_1",
"year": "2021"
},
{
"DOI": "10.1164/rccm.202105-1302OC",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_36_1"
},
{
"article-title": "Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID‐19",
"author": "Struyf T.",
"journal-title": "Cochrane Database of Systematic Reviews",
"key": "e_1_2_11_37_1",
"volume": "7",
"year": "2022"
},
{
"DOI": "10.1513/AnnalsATS.202011-1376CME",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_38_1"
},
{
"DOI": "10.1155/2019/4836378",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_39_1"
},
{
"DOI": "10.1016/j.arcmed.2020.06.007",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_40_1"
},
{
"DOI": "10.1016/j.scitotenv.2020.138277",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_41_1"
},
{
"author": "World Health Organization",
"key": "e_1_2_11_42_1",
"volume-title": "Living guidance for clinical management of COVID‐19: Living guidance, 23 November 2021",
"year": "2021"
},
{
"DOI": "10.1038/s42003-020-01190-y",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_43_1"
},
{
"DOI": "10.1080/14786419.2013.805331",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_44_1"
}
],
"reference-count": 43,
"references-count": 43,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1002/ptr.8047"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology"
],
"subtitle": [],
"title": "The influence of propolis plus <i>Hyoscyamus niger</i> L. against <scp>COVID</scp>‐19: A phase <scp>II</scp>, multicenter, placebo‐controlled, randomized trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy",
"volume": "38"
}